Human iPSC-derived mesenchymal stem cells encapsulated in PEGDA hydrogels mature into valve interstitial-like cells
- PMID: 29505894
- PMCID: PMC5907941
- DOI: 10.1016/j.actbio.2018.02.025
Human iPSC-derived mesenchymal stem cells encapsulated in PEGDA hydrogels mature into valve interstitial-like cells
Abstract
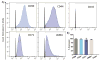
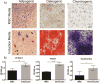
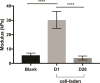
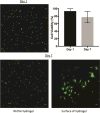
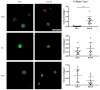
Despite recent advances in tissue engineered heart valves (TEHV), a major challenge is identifying a cell source for seeding TEHV scaffolds. Native heart valves are durable because valve interstitial cells (VICs) maintain tissue homeostasis by synthesizing and remodeling the extracellular matrix. This study demonstrates that induced pluripotent stem cells (iPSC)-derived mesenchymal stem cells (iMSCs) can be derived from iPSCs using a feeder-free protocol and then further matured into VICs by encapsulation within 3D hydrogels. The differentiation efficiency was characterized using flow cytometry, immunohistochemistry staining, and trilineage differentiation. Using our feeder-free differentiation protocol, iMSCs were differentiated from iPSCs and had CD90+, CD44+, CD71+, αSMA+, and CD45- expression. Furthermore, iMSCs underwent trilineage differentiation when cultured in induction media for 21 days. iMSCs were then encapsulated in poly(ethylene glycol)diacrylate (PEGDA) hydrogels grafted with adhesion peptide (RGDS) to promote remodeling and further maturation into VIC-like cells. VIC phenotype was assessed by the expression of alpha-smooth muscle actin (αSMA), vimentin, and collagen production after 28 days. When MSC-derived cells were encapsulated in PEGDA hydrogels that mimic the leaflet modulus, a decrease in αSMA expression and increase in vimentin was observed. In addition, iMSCs synthesized collagen type I after 28 days in 3D hydrogel culture. Thus, the results from this study suggest that iMSCs may be a promising cell source for TEHV.
Statement of significance: Developing a suitable cell source is a critical component for the success and durability of tissue engineered heart valves. The significance of this study is the generation of iPSCs-derived mesenchymal stem cells (iMSCs) that have the capacity to mature into valve interstitial-like cells when introduced into a 3D cell culture designed to mimic the layers of the valve leaflet. iMSCs were generated using a feeder-free protocol, which is one major advantage over other methods, as it is more clinically relevant. In addition to generating a potential new cell source for heart valve tissue engineering, this study also highlights the importance of a 3D culture environment to influence cell phenotype and function.
Keywords: Hydrogel; Induced pluripotent stem cells; Mesenchymal stem cells; PEG; Tissue engineering heart valves.
Copyright © 2018 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures



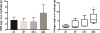
Similar articles
-
Biodegradable Poly-ε-Caprolactone Scaffolds with ECFCs and iMSCs for Tissue-Engineered Heart Valves.Int J Mol Sci. 2022 Jan 4;23(1):527. doi: 10.3390/ijms23010527. Int J Mol Sci. 2022. PMID: 35008953 Free PMC article.
-
Fn14 promotes differentiation of human mesenchymal stem cells into heart valvular interstitial cells by phenotypic characterization.J Cell Physiol. 2014 May;229(5):580-7. doi: 10.1002/jcp.24480. J Cell Physiol. 2014. PMID: 24122208
-
Role of cell-matrix interactions on VIC phenotype and tissue deposition in 3D PEG hydrogels.J Tissue Eng Regen Med. 2016 Oct;10(10):E443-E453. doi: 10.1002/term.1836. Epub 2013 Oct 16. J Tissue Eng Regen Med. 2016. PMID: 24130082 Free PMC article.
-
hiPSC-derived iMSCs: NextGen MSCs as an advanced therapeutically active cell resource for regenerative medicine.J Cell Mol Med. 2016 Aug;20(8):1571-88. doi: 10.1111/jcmm.12839. Epub 2016 Apr 21. J Cell Mol Med. 2016. PMID: 27097531 Free PMC article. Review.
-
Induced pluripotent stem cell-derived mesenchymal stem cells: whether they can become new stars of cell therapy.Stem Cell Res Ther. 2024 Oct 16;15(1):367. doi: 10.1186/s13287-024-03968-x. Stem Cell Res Ther. 2024. PMID: 39415276 Free PMC article. Review.
Cited by
-
Visible-Light Stiffness Patterning of GelMA Hydrogels Towards In Vitro Scar Tissue Models.Front Cell Dev Biol. 2022 Jul 5;10:946754. doi: 10.3389/fcell.2022.946754. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35865624 Free PMC article.
-
The Current Proceedings of PSC-Based Liver Fibrosis Therapy.Stem Cell Rev Rep. 2023 Oct;19(7):2155-2165. doi: 10.1007/s12015-023-10592-4. Epub 2023 Jul 25. Stem Cell Rev Rep. 2023. PMID: 37490204 Review.
-
Biodegradable and Non-Biodegradable Biomaterials and Their Effect on Cell Differentiation.Int J Mol Sci. 2022 Dec 19;23(24):16185. doi: 10.3390/ijms232416185. Int J Mol Sci. 2022. PMID: 36555829 Free PMC article. Review.
-
Changing Metabolism in Differentiating Cardiac Progenitor Cells-Can Stem Cells Become Metabolically Flexible Cardiomyocytes?Front Cardiovasc Med. 2018 Sep 19;5:119. doi: 10.3389/fcvm.2018.00119. eCollection 2018. Front Cardiovasc Med. 2018. PMID: 30283788 Free PMC article. Review.
-
Water-Soluble Photoinitiators in Biomedical Applications.Polymers (Basel). 2020 May 7;12(5):1073. doi: 10.3390/polym12051073. Polymers (Basel). 2020. PMID: 32392892 Free PMC article. Review.
References
-
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, C. American Heart Association Statistics, S. Stroke Statistics Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. - PMC - PubMed
-
- Jana S, Lerman A. Bioprinting a cardiac valve. Biotechnol Adv. 2015;33(8):1503–21. - PubMed
-
- Takkenberg JJ, Rajamannan NM, Rosenhek R, Kumar AS, Carapetis JR, Yacoub MH, D. Society for Heart Valve The need for a global perspective on heart valve disease epidemiology. The SHVD working group on epidemiology of heart valve disease founding statement. The Journal of heart valve disease. 2008;17(1):135–9. - PubMed
-
- Marijon E, Mirabel M, Celermajer DS, Jouven X. Rheumatic heart disease. Lancet. 2012;379(9819):953–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

