Mechanochemical coupling and bi-phasic force-velocity dependence in the ultra-fast ring ATPase SpoIIIE
- PMID: 29504934
- PMCID: PMC5858925
- DOI: 10.7554/eLife.32354
Mechanochemical coupling and bi-phasic force-velocity dependence in the ultra-fast ring ATPase SpoIIIE
Abstract
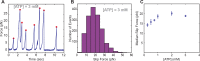
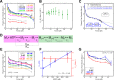
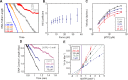
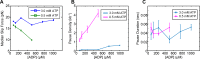
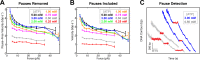
Multi-subunit ring-shaped ATPases are molecular motors that harness chemical free energy to perform vital mechanical tasks such as polypeptide translocation, DNA unwinding, and chromosome segregation. Previously we reported the intersubunit coordination and stepping behavior of the hexameric ring-shaped ATPase SpoIIIE (Liu et al., 2015). Here we use optical tweezers to characterize the motor's mechanochemistry. Analysis of the motor response to external force at various nucleotide concentrations identifies phosphate release as the likely force-generating step. Analysis of SpoIIIE pausing indicates that pauses are off-pathway events. Characterization of SpoIIIE slipping behavior reveals that individual motor subunits engage DNA upon ATP binding. Furthermore, we find that SpoIIIE's velocity exhibits an intriguing bi-phasic dependence on force. We hypothesize that this behavior is an adaptation of ultra-fast motors tasked with translocating DNA from which they must also remove DNA-bound protein roadblocks. Based on these results, we formulate a comprehensive mechanochemical model for SpoIIIE.
Keywords: B. subtilis; Ftsk/SpoIIIE; Ring ATPase; biochemistry; chemical biology; mechanochemistry; molecular biophysics; optical tweezers; single-molecule; structural biology.
© 2018, Liu et al.
Conflict of interest statement
NL, GC, YC, CB No competing interests declared
Figures



Similar articles
-
Two-subunit DNA escort mechanism and inactive subunit bypass in an ultra-fast ring ATPase.Elife. 2015 Oct 9;4:e09224. doi: 10.7554/eLife.09224. Elife. 2015. PMID: 26452092 Free PMC article.
-
Structure and DNA-binding properties of the Bacillus subtilis SpoIIIE DNA translocase revealed by single-molecule and electron microscopies.Nucleic Acids Res. 2014 Feb;42(4):2624-36. doi: 10.1093/nar/gkt1231. Epub 2013 Dec 1. Nucleic Acids Res. 2014. PMID: 24297254 Free PMC article.
-
Rebuilt AAA + motors reveal operating principles for ATP-fuelled machines.Nature. 2005 Oct 20;437(7062):1115-20. doi: 10.1038/nature04031. Nature. 2005. PMID: 16237435
-
Membrane-associated nanomotors for macromolecular transport.Curr Opin Biotechnol. 2012 Aug;23(4):537-44. doi: 10.1016/j.copbio.2011.11.031. Epub 2011 Dec 19. Curr Opin Biotechnol. 2012. PMID: 22189002 Review.
-
Pushing, pulling and trapping--modes of motor protein supported protein translocation.FEBS Lett. 2007 Jun 19;581(15):2820-8. doi: 10.1016/j.febslet.2007.04.015. Epub 2007 Apr 18. FEBS Lett. 2007. PMID: 17466297 Review.
Cited by
-
Optical tweezers in single-molecule biophysics.Nat Rev Methods Primers. 2021;1:25. doi: 10.1038/s43586-021-00021-6. Epub 2021 Mar 25. Nat Rev Methods Primers. 2021. PMID: 34849486 Free PMC article.
-
Effects of Ligand Binding on the Energy Landscape of Acyl-CoA-Binding Protein.Biophys J. 2020 Nov 3;119(9):1821-1832. doi: 10.1016/j.bpj.2020.09.016. Epub 2020 Sep 24. Biophys J. 2020. PMID: 33080224 Free PMC article.
-
Milestones in Bacillus subtilis sporulation research.Microb Cell. 2020 Nov 27;8(1):1-16. doi: 10.15698/mic2021.01.739. Microb Cell. 2020. PMID: 33490228 Free PMC article. Review.
-
Membrane fission during bacterial spore development requires cellular inflation driven by DNA translocation.Curr Biol. 2022 Oct 10;32(19):4186-4200.e8. doi: 10.1016/j.cub.2022.08.014. Epub 2022 Aug 29. Curr Biol. 2022. PMID: 36041438 Free PMC article.
-
Dynamic ParB-DNA interactions initiate and maintain a partition condensate for bacterial chromosome segregation.Nucleic Acids Res. 2023 Nov 27;51(21):11856-11875. doi: 10.1093/nar/gkad868. Nucleic Acids Res. 2023. PMID: 37850647 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

