TAK1 mediates excessive autophagy via p38 and ERK in cisplatin-induced acute kidney injury
- PMID: 29504713
- PMCID: PMC5908118
- DOI: 10.1111/jcmm.13585
TAK1 mediates excessive autophagy via p38 and ERK in cisplatin-induced acute kidney injury
Abstract
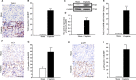
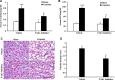
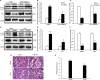
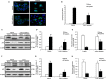
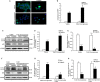
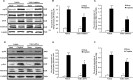
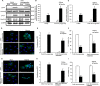
The ability of cisplatin (cis-diamminedichloroplatinum II) toxicity to induce acute kidney injury (AKI) has attracted people's attention and concern for a long time, but its molecular mechanisms are still widely unknown. We found that the expression of transforming growth factor-β (TGF-β)-activated kinase 1 (TAK1) could be increased in kidneys of mice administrated with cisplatin. Autophagy is an evolutionarily conserved catabolic pathway and is involved in various acute and chronic injuries. Moreover, p38 MAPK (mitogen-activated protein kinase) and ERK regulate autophagy in response to various stimuli. Therefore, our hypothesis is that cisplatin activates TAK1, which phosphorylates p38 and ERK, leading to excessive autophagy of tubular epithelial cells and thus exacerbating kidney damage. Here, BALB/c mice were intraperitoneally injected with a TAK1 inhibitor and were then administrated with sham or cisplatin at 20 mg/kg by intraperitoneal injection. Compared with mice in the vehicle cisplatin group, mice intraperitoneally injected with a TAK1 inhibitor were found to have lower serum creatinine and less tubular damage following cisplatin-induced AKI. Furthermore, inhibition of TAK1 reduced p38 and Erk phosphorylation, decreased expression of LC3II and reversed the down-regulation of P62 expression induced by cisplatin. The hypothesis was verified with tubular epithelial cells administrated with cisplatin in vitro. Finally, p38 inhibitor or ERK inhibitor abated autophagy activation and cell viability reduction in tubular epithelial cells treated with cisplatin plus TAK1 overexpression vector. Taken together, our results show that cisplatin activates TAK1, which phosphorylates p38 and ERK, leading to excessive autophagy of tubular epithelial cells that exacerbates kidney damage.
Keywords: ERK; TAK1; acute kidney injury; autophagy; p38.
© 2018 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

Similar articles
-
TAK1 mediates apoptosis via p38 involve in ischemia-induced renal fibrosis.Artif Cells Nanomed Biotechnol. 2018;46(sup1):1016-1025. doi: 10.1080/21691401.2018.1442841. Epub 2018 Apr 16. Artif Cells Nanomed Biotechnol. 2018. PMID: 29661023
-
TAK1 deficiency attenuates cisplatin-induced acute kidney injury.Am J Physiol Renal Physiol. 2020 Jan 1;318(1):F209-F215. doi: 10.1152/ajprenal.00516.2019. Epub 2019 Dec 9. Am J Physiol Renal Physiol. 2020. PMID: 31813254 Free PMC article.
-
Dual-specificity phosphatase 26 protects against kidney injury caused by ischaemia-reperfusion through restraint of TAK1-JNK/p38-mediated apoptosis and inflammation of renal tubular epithelial cells.Toxicol Appl Pharmacol. 2024 Jun;487:116954. doi: 10.1016/j.taap.2024.116954. Epub 2024 May 4. Toxicol Appl Pharmacol. 2024. PMID: 38705402
-
AMPK/mTOR Signaling in Autophagy Regulation During Cisplatin-Induced Acute Kidney Injury.Front Physiol. 2020 Dec 17;11:619730. doi: 10.3389/fphys.2020.619730. eCollection 2020. Front Physiol. 2020. PMID: 33391038 Free PMC article. Review.
-
MAP3K kinases and kidney injury.Nefrologia (Engl Ed). 2019 Nov-Dec;39(6):568-580. doi: 10.1016/j.nefro.2019.03.004. Epub 2019 Jun 10. Nefrologia (Engl Ed). 2019. PMID: 31196660 Review. English, Spanish.
Cited by
-
Protein phosphatase 6 is a key factor regulating spermatogenesis.Cell Death Differ. 2020 Jun;27(6):1952-1964. doi: 10.1038/s41418-019-0472-9. Epub 2019 Dec 9. Cell Death Differ. 2020. PMID: 31819157 Free PMC article.
-
Chemotherapy-induced nephrotoxicity was improved by crocin in mouse model.Eur J Histochem. 2022 Oct 3;66(4):3541. doi: 10.4081/ejh.2022.3541. Eur J Histochem. 2022. PMID: 36190398 Free PMC article.
-
Tripartite Motif 8 Deficiency Relieves Hepatic Ischaemia/reperfusion Injury via TAK1-dependent Signalling Pathways.Int J Biol Sci. 2019 Jun 4;15(8):1618-1629. doi: 10.7150/ijbs.33323. eCollection 2019. Int J Biol Sci. 2019. PMID: 31360105 Free PMC article.
-
Dietary Chrysin Suppresses Formation of Actin Cytoskeleton and Focal Adhesion in AGE-Exposed Mesangial Cells and Diabetic Kidney: Role of Autophagy.Nutrients. 2019 Jan 9;11(1):127. doi: 10.3390/nu11010127. Nutrients. 2019. PMID: 30634545 Free PMC article.
-
Microglial-specific depletion of TAK1 is neuroprotective in the acute phase after ischemic stroke.J Mol Med (Berl). 2020 Jun;98(6):833-847. doi: 10.1007/s00109-020-01916-9. Epub 2020 May 7. J Mol Med (Berl). 2020. PMID: 32382778 Free PMC article.
References
-
- Bellmunt J, Pons F, Orsola A. Molecular determinants of response to cisplatin‐based neoadjuvant chemotherapy. Curr Opin Urol. 2013;23:466‐471. - PubMed
-
- Galluzzi L, Vicencio JM, Kepp O, Tasdemir E, Maiuri MC, Kroemer G. To die or not to die: that is the autophagic question. Curr Mol Med. 2008;8:78‐91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

