Design of a phase IV randomised, double-blind, placebo-controlled trial assessing the Im P act of R esidual Inflammation Detected via Imaging T E chniques, D rug Levels and Patient Characteristics on the Outcome of Dose Taper I ng of Adalimumab in C linical Remission Rheumatoid Ar T hritis (RA) patients (PREDICTRA)
- PMID: 29490959
- PMCID: PMC5855387
- DOI: 10.1136/bmjopen-2017-019007
Design of a phase IV randomised, double-blind, placebo-controlled trial assessing the Im P act of R esidual Inflammation Detected via Imaging T E chniques, D rug Levels and Patient Characteristics on the Outcome of Dose Taper I ng of Adalimumab in C linical Remission Rheumatoid Ar T hritis (RA) patients (PREDICTRA)
Abstract
Introduction: The current American College of Rheumatology and European League Against Rheumatism treatment recommendations advise tapering biological disease-modifying antirheumatic drug (bDMARD) therapy in patients with rheumatoid arthritis (RA) who achieve stable clinical remission while receiving bDMARDs. However, not all patients maintain remission or low disease activity after tapering or discontinuation of bDMARDs. The aim of the ImPact of Residual Inflammation Detected via Imaging TEchniques, Drug Levels and Patient Characteristics on the Outcome of Dose TaperIng of Adalimumab in Clinical Remission Rheumatoid ArThritis (RA) study, or PREDICTRA, is to generate data on patient and disease characteristics that may predict the clinical course of a fixed dose-tapering regimen with the bDMARD adalimumab.
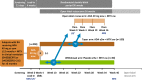
Methods and analysis: PREDICTRA is an ongoing, multicentre, phase IV, randomised, double-blind, parallel-group study of adalimumab dose tapering controlled by withdrawal in participants with RA who achieved stable clinical remission while receiving adalimumab. The study includes a screening period, a 4-week lead-in period with open-label adalimumab 40 mg every other week and a subsequent 36-week double-blind period during which participants are randomised 5:1 to adalimumab 40 mg every 3 weeks (taper arm) or placebo (withdrawal arm). The primary explanatory efficacy variables are lead-in baseline hand and wrist MRI-detected synovitis and bone marrow oedema scores, as well as a composite of both scores; the dependent variable is the occurrence of flare up to week 40. Additional efficacy variables, safety, pharmacokinetics, biomarkers and immunogenicity will also be assessed, and an ultrasound substudy will be conducted.
Ethics and dissemination: The study is conducted in accordance with the International Conference on Harmonisation guidelines, local laws and the ethical principles of the Declaration of Helsinki. All participants are required to sign a written informed consent statement before the start of any study procedures.
Trial registration number: EudraCT 2014-001114-26 and NCT02198651; Pre-results.
Keywords: adalimumab; discontinuation; rheumatoid arthritis; rheumatology; tapering; withdrawal.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: PE has received research grants and/or consulting fees from AbbVie, Bristol-Myers Squibb, Lilly, Merck, Novartis, Pfizer, Roche, Sandoz and UCB. GRB has received research grants and/or consulting fees from AbbVie, Bristol-Myers Squibb, Lilly, MSD, Novartis, Pfizer, Roche, Sandoz and UCB. EN has received speaker fees from AbbVie, Roche, Bristol-Myers Squibb, Pfizer, UCB, Lilly, Novartis, Janssen and Celgene GmbH and honoraria from AbbVie. YZ and MH are full-time employees of AbbVie and may hold AbbVie stock or stock options. PGC has received speakers’ bureau or consulting fees from AbbVie, Bristol- Myers Squibb, Lilly, Novartis, Pfizer and Roche.
Figures
Similar articles
-
Adalimumab dose tapering in patients with rheumatoid arthritis who are in long-standing clinical remission: results of the phase IV PREDICTRA study.Ann Rheum Dis. 2020 Aug;79(8):1023-1030. doi: 10.1136/annrheumdis-2020-217246. Epub 2020 May 13. Ann Rheum Dis. 2020. PMID: 32404343 Free PMC article. Clinical Trial.
-
Adalimumab added to a treat-to-target strategy with methotrexate and intra-articular triamcinolone in early rheumatoid arthritis increased remission rates, function and quality of life. The OPERA Study: an investigator-initiated, randomised, double-blind, parallel-group, placebo-controlled trial.Ann Rheum Dis. 2014 Apr;73(4):654-61. doi: 10.1136/annrheumdis-2012-202735. Epub 2013 Feb 23. Ann Rheum Dis. 2014. PMID: 23434570 Clinical Trial.
-
[Adalimumab plus methotrexate for the treatment of rheumatoid arthritis: a multi-center randomized, double-blind, placebo-controlled clinical study.].Zhonghua Nei Ke Za Zhi. 2009 Nov;48(11):916-21. Zhonghua Nei Ke Za Zhi. 2009. PMID: 20079321 Clinical Trial. Chinese.
-
Tapering biologics in rheumatoid arthritis: a pragmatic approach for clinical practice.Clin Rheumatol. 2017 Jan;36(1):1-8. doi: 10.1007/s10067-016-3490-8. Epub 2016 Nov 28. Clin Rheumatol. 2017. PMID: 27896522 Review.
-
Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation.Health Technol Assess. 2016 Apr;20(35):1-610. doi: 10.3310/hta20350. Health Technol Assess. 2016. PMID: 27140438 Free PMC article. Review.
Cited by
-
What Is the Clinical Relevance of TNF Inhibitor Immunogenicity in the Management of Patients With Rheumatoid Arthritis?Front Immunol. 2020 Apr 7;11:589. doi: 10.3389/fimmu.2020.00589. eCollection 2020. Front Immunol. 2020. PMID: 32318070 Free PMC article. Review.
-
The effect of deep or sustained remission on maintenance of remission after dose reduction or withdrawal of etanercept in patients with rheumatoid arthritis.Arthritis Res Ther. 2019 Jul 5;21(1):164. doi: 10.1186/s13075-019-1937-4. Arthritis Res Ther. 2019. PMID: 31277720 Free PMC article.
-
Down-titration and discontinuation strategies of tumour necrosis factor-blocking agents for rheumatoid arthritis in patients with low disease activity.Cochrane Database Syst Rev. 2019 May 24;5(5):CD010455. doi: 10.1002/14651858.CD010455.pub3. Cochrane Database Syst Rev. 2019. PMID: 31125448 Free PMC article.
-
Adalimumab dose tapering in patients with rheumatoid arthritis who are in long-standing clinical remission: results of the phase IV PREDICTRA study.Ann Rheum Dis. 2020 Aug;79(8):1023-1030. doi: 10.1136/annrheumdis-2020-217246. Epub 2020 May 13. Ann Rheum Dis. 2020. PMID: 32404343 Free PMC article. Clinical Trial.
References
-
- Gibofsky A. Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am J Manag Care 2012;18:S295–302. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
