The post-translational modification, SUMOylation, and cancer (Review)
- PMID: 29484374
- PMCID: PMC5843405
- DOI: 10.3892/ijo.2018.4280
The post-translational modification, SUMOylation, and cancer (Review)
Abstract
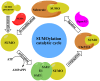
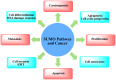
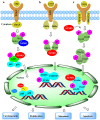
SUMOylation is a reversible post-translational modification which has emerged as a crucial molecular regulatory mechanism, involved in the regulation of DNA damage repair, immune responses, carcinogenesis, cell cycle progression and apoptosis. Four SUMO isoforms have been identified, which are SUMO1, SUMO2/3 and SUMO4. The small ubiquitin-like modifier (SUMO) pathway is conserved in all eukaryotes and plays pivotal roles in the regulation of gene expression, cellular signaling and the maintenance of genomic integrity. The SUMO catalytic cycle includes maturation, activation, conjugation, ligation and de-modification. The dysregulation of the SUMO system is associated with a number of diseases, particularly cancer. SUMOylation is widely involved in carcinogenesis, DNA damage response, cancer cell proliferation, metastasis and apoptosis. SUMO can be used as a potential therapeutic target for cancer. In this review, we briefly outline the basic concepts of the SUMO system and summarize the involvement of SUMO proteins in cancer cells in order to better understand the role of SUMO in human disease.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
SUMOylation in carcinogenesis.Cancer Lett. 2012 Mar 28;316(2):113-25. doi: 10.1016/j.canlet.2011.10.036. Epub 2011 Nov 2. Cancer Lett. 2012. PMID: 22138131 Review.
-
Mapping the SUMOylated landscape.FEBS J. 2015 Oct;282(19):3669-80. doi: 10.1111/febs.13378. Epub 2015 Jul 31. FEBS J. 2015. PMID: 26185901 Free PMC article. Review.
-
Assessing the Role of Paralog-Specific Sumoylation of HDAC1.Methods Mol Biol. 2017;1510:329-337. doi: 10.1007/978-1-4939-6527-4_24. Methods Mol Biol. 2017. PMID: 27761832
-
Identification of small ubiquitin-like modifier substrates with diverse functions using the Xenopus egg extract system.Mol Cell Proteomics. 2014 Jul;13(7):1659-75. doi: 10.1074/mcp.M113.035626. Epub 2014 May 5. Mol Cell Proteomics. 2014. PMID: 24797264 Free PMC article.
-
E2-mediated small ubiquitin-like modifier (SUMO) modification of thymine DNA glycosylase is efficient but not selective for the enzyme-product complex.J Biol Chem. 2014 May 30;289(22):15810-9. doi: 10.1074/jbc.M114.572081. Epub 2014 Apr 21. J Biol Chem. 2014. PMID: 24753249 Free PMC article.
Cited by
-
Dysregulated gene expression of SUMO machinery components induces the resistance to anti-PD-1 immunotherapy in lung cancer by upregulating the death of peripheral blood lymphocytes.Front Immunol. 2024 Aug 15;15:1424393. doi: 10.3389/fimmu.2024.1424393. eCollection 2024. Front Immunol. 2024. PMID: 39211047 Free PMC article.
-
SUMO3 modification by PIAS1 modulates androgen receptor cellular distribution and stability.Cell Commun Signal. 2019 Nov 21;17(1):153. doi: 10.1186/s12964-019-0457-9. Cell Commun Signal. 2019. PMID: 31752909 Free PMC article.
-
The SUMOylation and ubiquitination crosstalk in cancer.J Cancer Res Clin Oncol. 2023 Nov;149(17):16123-16146. doi: 10.1007/s00432-023-05310-z. Epub 2023 Aug 28. J Cancer Res Clin Oncol. 2023. PMID: 37640846 Review.
-
SUMOylation regulation of ribosome biogenesis: Emerging roles for USP36.Front RNA Res. 2024;2:1389104. doi: 10.3389/frnar.2024.1389104. Epub 2024 Apr 3. Front RNA Res. 2024. PMID: 38764604 Free PMC article.
-
Proteome-wide and lysine crotonylation profiling reveals the importance of crotonylation in chrysanthemum (Dendranthema grandiforum) under low-temperature.BMC Genomics. 2021 Jan 14;22(1):51. doi: 10.1186/s12864-020-07365-5. BMC Genomics. 2021. PMID: 33446097 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

