Dual targeting of mitochondrial function and mTOR pathway as a therapeutic strategy for diffuse intrinsic pontine glioma
- PMID: 29484131
- PMCID: PMC5800923
- DOI: 10.18632/oncotarget.24045
Dual targeting of mitochondrial function and mTOR pathway as a therapeutic strategy for diffuse intrinsic pontine glioma
Abstract
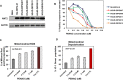
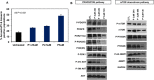
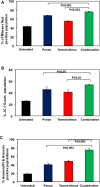
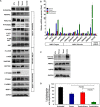
Diffuse Intrinsic Pontine Gliomas (DIPG) are the most devastating of all pediatric brain tumors. They mostly affect young children and, as there are no effective treatments, almost all patients with DIPG will die of their tumor within 12 months of diagnosis. A key feature of this devastating tumor is its intrinsic resistance to all clinically available therapies. It has been shown that glioma development is associated with metabolic reprogramming, redox state disruption and resistance to apoptotic pathways. The mitochondrion is an attractive target as a key organelle that facilitates these critical processes. PENAO is a novel anti-cancer compound that targets mitochondrial function by inhibiting adenine nucleotide translocase (ANT). Here we found that DIPG neurosphere cultures express high levels of ANT2 protein and are sensitive to the mitochondrial inhibitor PENAO through oxidative stress, while its apoptotic effects were found to be further enhanced upon co-treatment with mTOR inhibitor temsirolimus. This combination therapy was found to act through inhibition of PI3K/AKT/mTOR pathway, HSP90 and activation of AMPK. In vivo experiments employing an orthotopic model of DIPG showed a marginal anti-tumour effect likely due to poor penetration of the inhibitors into the brain. Further testing of this anti-DIPG strategy with compounds that penetrate the BBB is warranted.
Keywords: DIPG; PDGFR; mTOR; mitochondria; paediatric brain tumour.
Conflict of interest statement
CONFLICTS OF INTEREST No conflicts of interest.
Figures

Similar articles
-
Preclinical evaluation of convection-enhanced delivery of liposomal doxorubicin to treat pediatric diffuse intrinsic pontine glioma and thalamic high-grade glioma.J Neurosurg Pediatr. 2017 May;19(5):518-530. doi: 10.3171/2016.9.PEDS16152. Epub 2017 Feb 17. J Neurosurg Pediatr. 2017. PMID: 28291423
-
Combined use of CDK4/6 and mTOR inhibitors induce synergistic growth arrest of diffuse intrinsic pontine glioma cells via mutual downregulation of mTORC1 activity.Cancer Manag Res. 2018 Sep 12;10:3483-3500. doi: 10.2147/CMAR.S167095. eCollection 2018. Cancer Manag Res. 2018. PMID: 30254491 Free PMC article.
-
Dual Inhibition of PI3K/AKT and MEK/ERK Pathways Induces Synergistic Antitumor Effects in Diffuse Intrinsic Pontine Glioma Cells.Transl Oncol. 2017 Apr;10(2):221-228. doi: 10.1016/j.tranon.2016.12.008. Epub 2017 Feb 9. Transl Oncol. 2017. PMID: 28189993 Free PMC article.
-
Invaders Exposed: Understanding and Targeting Tumor Cell Invasion in Diffuse Intrinsic Pontine Glioma.Front Oncol. 2020 Feb 7;10:92. doi: 10.3389/fonc.2020.00092. eCollection 2020. Front Oncol. 2020. PMID: 32117746 Free PMC article. Review.
-
Intersection of Brain Development and Paediatric Diffuse Midline Gliomas: Potential Role of Microenvironment in Tumour Growth.Brain Sci. 2018 Nov 16;8(11):200. doi: 10.3390/brainsci8110200. Brain Sci. 2018. PMID: 30453529 Free PMC article. Review.
Cited by
-
Inhibition of mitochondrial translocase SLC25A5 and histone deacetylation is an effective combination therapy in neuroblastoma.Int J Cancer. 2023 Apr 1;152(7):1399-1413. doi: 10.1002/ijc.34349. Epub 2022 Nov 17. Int J Cancer. 2023. PMID: 36346110 Free PMC article.
-
International experience in the development of patient-derived xenograft models of diffuse intrinsic pontine glioma.J Neurooncol. 2019 Jan;141(2):253-263. doi: 10.1007/s11060-018-03038-2. Epub 2018 Nov 16. J Neurooncol. 2019. PMID: 30446898
-
Autophagy as a Potential Therapy for Malignant Glioma.Pharmaceuticals (Basel). 2020 Jul 19;13(7):156. doi: 10.3390/ph13070156. Pharmaceuticals (Basel). 2020. PMID: 32707662 Free PMC article. Review.
-
Dual targeting of histone deacetylases and MYC as potential treatment strategy for H3-K27M pediatric gliomas.Elife. 2024 Aug 2;13:RP96257. doi: 10.7554/eLife.96257. Elife. 2024. PMID: 39093942 Free PMC article.
-
Autophagic and Apoptotic Pathways as Targets for Chemotherapy in Glioblastoma.Int J Mol Sci. 2018 Nov 27;19(12):3773. doi: 10.3390/ijms19123773. Int J Mol Sci. 2018. PMID: 30486451 Free PMC article. Review.
References
-
- Wen PY, Kesari S. Malignant Gliomas in Adults. N Engl J Med. 2008;359:492–507. https://doi.org/10.1056/NEJMra0708126. - DOI - PubMed
-
- Buczkowicz P, Hawkins C. Pathology, Molecular Genetics, and Epigenetics of Diffuse Intrinsic Pontine Glioma. Front Oncol. 2015;5:147. https://doi.org/10.3389/fonc.2015.00147. - DOI - PMC - PubMed
-
- Panditharatna E, Yaeger K, Kilburn LB, Packer RJ, Nazarian J. Clinicopathology of diffuse intrinsic pontine glioma and its redefined genomic and epigenomic landscape. Cancer Genet. 2015;208:367–73. https://doi.org/10.1016/j.cancergen.2015.04.008. - DOI - PubMed
-
- Grill J, Puget S, Andreiuolo F, Philippe C, MacConaill L, Kieran MW. Critical oncogenic mutations in newly diagnosed pediatric diffuse intrinsic pontine glioma. Pediatr Blood Cancer. 2012;58:489–91. https://doi.org/10.1002/pbc.24060. - DOI - PubMed
-
- Wu G, Diaz AK, Paugh BS, Rankin SL, Ju B, Li Y, Zhu X, Qu C, Chen X, Zhang J, Easton J, Edmonson M, Ma X, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 2014;46:444–50. https://doi.org/10.1038/ng.2938. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

