Melatonin activates FIS1, DYN1, and DYN2 Plasmodium falciparum related-genes for mitochondria fission: Mitoemerald-GFP as a tool to visualize mitochondria structure
- PMID: 29480948
- PMCID: PMC6585791
- DOI: 10.1111/jpi.12484
Melatonin activates FIS1, DYN1, and DYN2 Plasmodium falciparum related-genes for mitochondria fission: Mitoemerald-GFP as a tool to visualize mitochondria structure
Erratum in
-
Corrigendum.J Pineal Res. 2019 Sep;67(2):e12595. doi: 10.1111/jpi.12595. Epub 2019 Jul 26. J Pineal Res. 2019. PMID: 31393648 Free PMC article. No abstract available.
Abstract
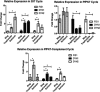
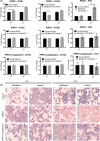
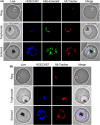
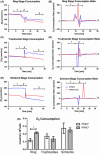
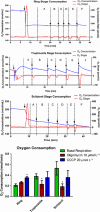
Malaria causes millions of deaths worldwide and is considered a huge burden to underdeveloped countries. The number of cases with resistance to all antimalarials is continuously increasing, making the identification of novel drugs a very urgent necessity. A potentially very interesting target for novel therapeutic intervention is the parasite mitochondrion. In this work, we studied in Plasmodium falciparum 3 genes coding for proteins homologues of the mammalian FIS1 (Mitochondrial Fission Protein 1) and DRP1 (Dynamin Related Protein 1) involved in mitochondrial fission. We studied the expression of P. falciparum genes that show ample sequence and structural homologies with the mammalian counterparts, namely FIS1, DYN1, and DYN2. The encoded proteins are characterized by a distinct pattern of expression throughout the erythrocytic cycle of P. falciparum, and their mRNAs are modulated by treating the parasite with the host hormone melatonin. We have previously reported that the knockout of the Plasmodium gene that codes for protein kinase 7 is essential for melatonin sensing. We here show that PfPk7 knockout results in major alterations of mitochondrial fission genes expression when compared to wild-type parasites, and no change in fission proteins expression upon treatment with the host hormone. Finally, we have compared the morphological characteristics (using MitoTracker Red CMX Ros) and oxygen consumption properties of P. falciparum mitochondria in wild-type parasites and PfPk7 Knockout strains. A novel GFP construct targeted to the mitochondrial matrix to wild-type parasites was also developed to visualize P. falciparum mitochondria. We here show that, the functional characteristics of P. falciparum are profoundly altered in cells lacking protein kinase 7, suggesting that this enzyme plays a major role in the control of mitochondrial morphogenesis and maturation during the intra-erythrocyte cell cycle progression.
Keywords: GFP; Plasmodium falciparum; malaria parasites; melatonin; mitochondria.
© 2018 The Authors. Journal of Pineal Research Published by John Wiley & Sons Ltd.
Figures

Similar articles
-
Dispensable Role of Mitochondrial Fission Protein 1 (Fis1) in the Erythrocytic Development of Plasmodium falciparum.mSphere. 2020 Sep 23;5(5):e00579-20. doi: 10.1128/mSphere.00579-20. mSphere. 2020. PMID: 32968006 Free PMC article.
-
Molecular basis of synchronous replication of malaria parasites in the blood stage.Curr Opin Microbiol. 2021 Oct;63:210-215. doi: 10.1016/j.mib.2021.08.002. Epub 2021 Aug 21. Curr Opin Microbiol. 2021. PMID: 34428626 Review.
-
Ubiquitin proteasome system and the atypical kinase PfPK7 are involved in melatonin signaling in Plasmodium falciparum.J Pineal Res. 2012 Sep;53(2):147-53. doi: 10.1111/j.1600-079X.2012.00981.x. Epub 2012 Feb 21. J Pineal Res. 2012. PMID: 22348509 Free PMC article.
-
Loss of MIEF1/MiD51 confers susceptibility to BAX-mediated cell death and PINK1-PRKN-dependent mitophagy.Autophagy. 2019 Dec;15(12):2107-2125. doi: 10.1080/15548627.2019.1596494. Epub 2019 Mar 28. Autophagy. 2019. PMID: 30894073 Free PMC article.
-
The Drp1-Mediated Mitochondrial Fission Protein Interactome as an Emerging Core Player in Mitochondrial Dynamics and Cardiovascular Disease Therapy.Int J Mol Sci. 2023 Mar 17;24(6):5785. doi: 10.3390/ijms24065785. Int J Mol Sci. 2023. PMID: 36982862 Free PMC article. Review.
Cited by
-
Decoding the Role of Melatonin Structure on Plasmodium falciparum Human Malaria Parasites Synchronization Using 2-Sulfenylindoles Derivatives.Biomolecules. 2022 Apr 26;12(5):638. doi: 10.3390/biom12050638. Biomolecules. 2022. PMID: 35625565 Free PMC article.
-
Mitochondrial quality control mechanisms as molecular targets in cardiac ischemia-reperfusion injury.Acta Pharm Sin B. 2020 Oct;10(10):1866-1879. doi: 10.1016/j.apsb.2020.03.004. Epub 2020 Apr 8. Acta Pharm Sin B. 2020. PMID: 33163341 Free PMC article. Review.
-
Malaria parasites and circadian rhythm: New insights into an old puzzle.Curr Res Microb Sci. 2020 Dec 14;2:100017. doi: 10.1016/j.crmicr.2020.100017. eCollection 2021 Dec. Curr Res Microb Sci. 2020. PMID: 34841309 Free PMC article.
-
Emerging insights into pulmonary hypertension: the potential role of mitochondrial dysfunction and redox homeostasis.Mol Cell Biochem. 2025 Mar;480(3):1407-1429. doi: 10.1007/s11010-024-05096-9. Epub 2024 Sep 10. Mol Cell Biochem. 2025. PMID: 39254871 Review.
-
Role of Melatonin in the Synchronization of Asexual Forms in the Parasite Plasmodium falciparum.Biomolecules. 2020 Aug 27;10(9):1243. doi: 10.3390/biom10091243. Biomolecules. 2020. PMID: 32867164 Free PMC article. Review.
References
-
- World Health Organization . World Malaria Report 2016; 2016.
-
- Mita T, Tanabe K. Evolution of Plasmodium falciparum drug resistance: implications for the development and containment of artemisinin resistance. Jpn J Infect Dis. 2012;65:465‐475. - PubMed
-
- Vaidya AB, Mather MW. Mitochondrial evolution and functions in malaria parasites. Annu Rev Microbiol. 2009;63:249‐267. - PubMed
-
- Painter HJ, Morrisey JM, Mather MW, Vaidya AB. Specific role of mitochondrial electron transport in blood‐stage Plasmodium falciparum . Nature. 2007;446:88‐91. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

