Evaluation of vaccine derived poliovirus type 2 outbreak response options: A randomized controlled trial, Karachi, Pakistan
- PMID: 29477307
- PMCID: PMC5869272
- DOI: 10.1016/j.vaccine.2018.02.051
Evaluation of vaccine derived poliovirus type 2 outbreak response options: A randomized controlled trial, Karachi, Pakistan
Abstract
Background: Outbreaks of circulating vaccine derived polioviruses type 2 (cVDPV2) remain a risk to poliovirus eradication in an era without live poliovirus vaccine containing type 2 in routine immunization. We evaluated existing outbreak response strategies recommended by the World Health Organization (WHO) for control of cVDPV2 outbreaks.
Methods: Seronegative children for poliovirus type 2 (PV2) at 22 weeks of life were assigned to one of four study groups and received respectively (1) one dose of trivalent oral poliovirus vaccine (tOPV); (2) monovalent OPV 2 (mOPV2); (3) tOPV together with a dose of inactivated poliovirus vaccine (IPV); or (4) mOPV2 with monovalent high-potency IPV type 2. Stool and blood samples were collected and assessed for presence of PV2 (stool) and anti-polio antibodies (sera).
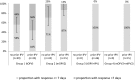
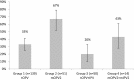
Results: We analyzed data from 265 children seronegative for PV2. Seroconversion to PV2 was achieved in 48, 76, 98 and 100% in Groups 1-4 respectively. mOPV2 was more immunogenic than tOPV alone (p < 0.001); and OPV in combination with IPV was more immunogenic than OPV alone (p < 0.001). There were 33%, 67%, 20% and 43% PV2 excretors in Groups 1-4 respectively. mOPV2 resulted in more prevalent shedding of PV2 than when tOPV was used (p < 0.001); and tOPV together with IPV resulted in lower excretion of PV2 than tOPV alone (p = 0.046).
Conclusion: mOPV2 was a more potent vaccine than tOPV. Adding IPV to OPV improved immunological response; adding IPV also seemed to have shortened the duration of PV2 shedding. mIPV2 did not provide measurable improvement of immune response when compared to conventional IPV. WHO recommendation to use mOPV2 as a vaccine of first choice in cVDPV2 outbreak response was supported by our findings. Clinical Trial registry number: NCT02189811.
Keywords: Polio outbreak response; Polio vaccines; Polio virus 2.
Copyright © 2018 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
Similar articles
-
Risk factors for the spread of vaccine-derived type 2 polioviruses after global withdrawal of trivalent oral poliovirus vaccine and the effects of outbreak responses with monovalent vaccine: a retrospective analysis of surveillance data for 51 countries in Africa.Lancet Infect Dis. 2022 Feb;22(2):284-294. doi: 10.1016/S1473-3099(21)00453-9. Epub 2021 Oct 11. Lancet Infect Dis. 2022. PMID: 34648733 Free PMC article.
-
Does Simultaneous Administration of Bivalent (Types 1 and 3) Oral Poliovirus Vaccine and Inactivated Poliovirus Vaccine Induce Mucosal Cross-immunity to Poliovirus Type 2?Clin Infect Dis. 2018 Oct 30;67(suppl_1):S51-S56. doi: 10.1093/cid/ciy604. Clin Infect Dis. 2018. PMID: 30376088 Free PMC article. Clinical Trial.
-
Immunogenicity of a new routine vaccination schedule for global poliomyelitis prevention: an open-label, randomised controlled trial.Lancet. 2015 Dec 12;386(10011):2413-21. doi: 10.1016/S0140-6736(15)00237-8. Epub 2015 Sep 18. Lancet. 2015. PMID: 26388534 Clinical Trial.
-
Oral and inactivated poliovirus vaccines in the newborn: a review.Vaccine. 2013 May 17;31(21):2517-24. doi: 10.1016/j.vaccine.2012.06.020. Epub 2012 Jun 20. Vaccine. 2013. PMID: 22728224 Free PMC article. Review.
-
Immunogenicity of New Primary Immunization Schedules With Inactivated Poliovirus Vaccine and Bivalent Oral Polio Vaccine for the Polio Endgame: A Review.Clin Infect Dis. 2018 Oct 30;67(suppl_1):S35-S41. doi: 10.1093/cid/ciy633. Clin Infect Dis. 2018. PMID: 30376081 Free PMC article. Review.
Cited by
-
Immunogenicity of Reduced-Dose Monovalent Type 2 Oral Poliovirus Vaccine in Mocuba, Mozambique.J Infect Dis. 2022 Aug 24;226(2):292-298. doi: 10.1093/infdis/jiaa704. J Infect Dis. 2022. PMID: 33180924 Free PMC article. Clinical Trial.
-
Modeling the spread of circulating vaccine-derived poliovirus type 2 outbreaks and interventions: A case study of Nigeria.Vaccine X. 2024 Mar 16;18:100476. doi: 10.1016/j.jvacx.2024.100476. eCollection 2024 Jun. Vaccine X. 2024. PMID: 38617838 Free PMC article.
-
Deployment of novel oral polio vaccine type 2 under emergency use listing in Nigeria: the rollout experience.Pan Afr Med J. 2023 Jul 13;45(Suppl 2):3. doi: 10.11604/pamj.supp.2023.45.2.38033. eCollection 2023. Pan Afr Med J. 2023. PMID: 38370105 Free PMC article. Review.
-
Risk factors for the spread of vaccine-derived type 2 polioviruses after global withdrawal of trivalent oral poliovirus vaccine and the effects of outbreak responses with monovalent vaccine: a retrospective analysis of surveillance data for 51 countries in Africa.Lancet Infect Dis. 2022 Feb;22(2):284-294. doi: 10.1016/S1473-3099(21)00453-9. Epub 2021 Oct 11. Lancet Infect Dis. 2022. PMID: 34648733 Free PMC article.
-
A circulating vaccine-derived poliovirus type 2 outbreak in a chronic conflict setting: a descriptive epidemiological study in South Sudan - 2020 to 2021.BMC Infect Dis. 2023 Nov 21;23(1):816. doi: 10.1186/s12879-023-08758-z. BMC Infect Dis. 2023. PMID: 37990165 Free PMC article.
References
-
- Cases of Wild Poliovirus by Country and Year. Available at: <http://www.polioeradication.org/Dataandmonitoring/Poliothisweek/Wildpol.... [accessed 06/09/2017].
-
- Morales M., Tangermann R.H., Wassilak S.G. Progress toward polio eradication – worldwide, 2015–2016. MMWR Morb Mortal Wkly Rep. 2016;65:470–473. - PubMed
-
- Centers for Disease C, Prevention. Apparent global interruption of wild poliovirus type 2 transmission. MMWR Morb Mortal Wkly Rep 2001; 50:222–4. - PubMed
-
- Sutter R.W., Platt L., Mach O., Jafari H., Aylward R.B. The new polio eradication end game: rationale and supporting evidence. J Infect Dis. 2014;210(Suppl 1):S434–S438. - PubMed
-
- GPEI. Global switch in oral polio vaccines. Available at: <http://maps.who.int/OPV_switch/>. [accessed 07/06/2016].
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

