Histone deacetylase inhibitors protect against cisplatin-induced acute kidney injury by activating autophagy in proximal tubular cells
- PMID: 29476062
- PMCID: PMC5833747
- DOI: 10.1038/s41419-018-0374-7
Histone deacetylase inhibitors protect against cisplatin-induced acute kidney injury by activating autophagy in proximal tubular cells
Abstract
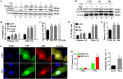
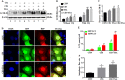
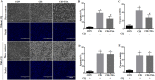
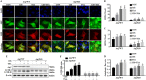
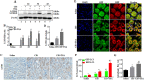
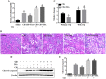
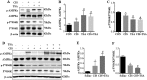
Histone deacetylase inhibitors (HDACi) have therapeutic effects in models of various renal diseases including acute kidney injury (AKI); however, the underlying mechanism remains unclear. Here we demonstrate that two widely tested HDACi (suberoylanilide hydroxamic acid (SAHA) and trichostatin A (TSA)) protect the kidneys in cisplatin-induced AKI by enhancing autophagy. In cultured renal proximal tubular cells, SAHA and TSA enhanced autophagy during cisplatin treatment. We further verified the protective effect of TSA against cisplatin-induced apoptosis in these cells. Notably, inhibition of autophagy by chloroquine or by autophagy gene 7 (Atg7) ablation diminished the protective effect of TSA. In mice, TSA increased autophagy in renal proximal tubules and protected against cisplatin-induced AKI. The in vivo effect of TSA was also abolished by chloroquine and by Atg7 knockout specifically from renal proximal tubules. Mechanistically, TSA stimulated AMPK and inactivated mTOR during cisplatin treatment of proximal tubule cells and kidneys in mice. Together, these results suggest that HDACi may protect kidneys by activating autophagy in proximal tubular cells.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Autophagy in Cisplatin Nephrotoxicity during Cancer Therapy.Cancers (Basel). 2021 Nov 10;13(22):5618. doi: 10.3390/cancers13225618. Cancers (Basel). 2021. PMID: 34830772 Free PMC article. Review.
-
Autophagy in proximal tubules protects against acute kidney injury.Kidney Int. 2012 Dec;82(12):1271-83. doi: 10.1038/ki.2012.261. Epub 2012 Aug 1. Kidney Int. 2012. PMID: 22854643 Free PMC article.
-
Lithium targeting of AMPK protects against cisplatin-induced acute kidney injury by enhancing autophagy in renal proximal tubular epithelial cells.FASEB J. 2019 Dec;33(12):14370-14381. doi: 10.1096/fj.201901712R. Epub 2019 Oct 29. FASEB J. 2019. PMID: 31661633
-
Inhibitors of histone deacetylases suppress cisplatin-induced p53 activation and apoptosis in renal tubular cells.Am J Physiol Renal Physiol. 2010 Feb;298(2):F293-300. doi: 10.1152/ajprenal.00410.2009. Epub 2009 Nov 4. Am J Physiol Renal Physiol. 2010. PMID: 19889954 Free PMC article.
-
Autophagy in kidney disease: Advances and therapeutic potential.Prog Mol Biol Transl Sci. 2020;172:107-133. doi: 10.1016/bs.pmbts.2020.01.008. Epub 2020 Feb 3. Prog Mol Biol Transl Sci. 2020. PMID: 32620239 Review.
Cited by
-
Histone deacetylase expression following cisplatin-induced acute kidney injury in male and female mice.Am J Physiol Renal Physiol. 2024 Oct 1;327(4):F623-F636. doi: 10.1152/ajprenal.00132.2024. Epub 2024 Aug 8. Am J Physiol Renal Physiol. 2024. PMID: 39116350
-
Epigenetic Mechanisms Involved in Cisplatin-Induced Nephrotoxicity: An Update.Pharmaceuticals (Basel). 2021 May 21;14(6):491. doi: 10.3390/ph14060491. Pharmaceuticals (Basel). 2021. PMID: 34063951 Free PMC article. Review.
-
3-Hydroxybutyrate Ameliorates the Progression of Diabetic Nephropathy.Antioxidants (Basel). 2022 Feb 14;11(2):381. doi: 10.3390/antiox11020381. Antioxidants (Basel). 2022. PMID: 35204263 Free PMC article.
-
Pharmacological inhibitors of autophagy have opposite effects in acute and chronic cisplatin-induced kidney injury.Am J Physiol Renal Physiol. 2022 Sep 1;323(3):F288-F298. doi: 10.1152/ajprenal.00097.2022. Epub 2022 Jul 7. Am J Physiol Renal Physiol. 2022. PMID: 35796459 Free PMC article.
-
Autophagy in Cisplatin Nephrotoxicity during Cancer Therapy.Cancers (Basel). 2021 Nov 10;13(22):5618. doi: 10.3390/cancers13225618. Cancers (Basel). 2021. PMID: 34830772 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

