Leucine-Rich Repeat Kinase 2 Controls the Ca2+/Nuclear Factor of Activated T Cells/IL-2 Pathway during Aspergillus Non-Canonical Autophagy in Dendritic Cells
- PMID: 29472933
- PMCID: PMC5809498
- DOI: 10.3389/fimmu.2018.00210
Leucine-Rich Repeat Kinase 2 Controls the Ca2+/Nuclear Factor of Activated T Cells/IL-2 Pathway during Aspergillus Non-Canonical Autophagy in Dendritic Cells
Abstract
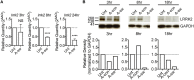
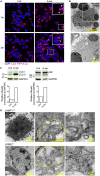
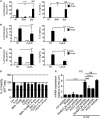
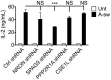
The Parkinson's disease-associated protein, Leucine-rich repeat kinase 2 (LRRK2), a known negative regulator of nuclear factor of activated T cells (NFAT), is expressed in myeloid cells such as macrophages and dendritic cells (DCs) and is involved in the host immune response against pathogens. Since, the Ca2+/NFAT/IL-2 axis has been previously found to regulate DC response to the fungus Aspergillus, we have investigated the role played by the kinase LRRK2 during fungal infection. Mechanistically, we found that in the early stages of the non-canonical autophagic response of DCs to the germinated spores of Aspergillus, LRRK2 undergoes progressive degradation and regulates NFAT translocation from the cytoplasm to the nucleus. Our results shed new light on the complexity of the Ca2+/NFAT/IL-2 pathway, where LRRK2 plays a role in controlling the immune response of DCs to Aspergillus.
Keywords: Aspergillus; NRON; autophagy; dendritic cell; leucine-rich repeat kinase 2; nuclear factor of activated T cells.
Figures


Similar articles
-
Leucine-rich repeat kinase 2 regulates mouse dendritic cell migration by ORAI2.FASEB J. 2019 Sep;33(9):9775-9784. doi: 10.1096/fj.201802550R. Epub 2019 Jun 5. FASEB J. 2019. PMID: 31166814
-
Leucine-Rich Repeat Kinase 2 Controls Inflammatory Cytokines Production through NF-κB Phosphorylation and Antigen Presentation in Bone Marrow-Derived Dendritic Cells.Int J Mol Sci. 2020 Mar 10;21(5):1890. doi: 10.3390/ijms21051890. Int J Mol Sci. 2020. PMID: 32164260 Free PMC article.
-
An increase in LRRK2 suppresses autophagy and enhances Dectin-1-induced immunity in a mouse model of colitis.Sci Transl Med. 2018 Jun 6;10(444):eaan8162. doi: 10.1126/scitranslmed.aan8162. Sci Transl Med. 2018. PMID: 29875204 Free PMC article.
-
LRRK2 and Autophagy.Adv Neurobiol. 2017;14:89-105. doi: 10.1007/978-3-319-49969-7_5. Adv Neurobiol. 2017. PMID: 28353280 Review.
-
LRRK2 and the Immune System.Adv Neurobiol. 2017;14:123-143. doi: 10.1007/978-3-319-49969-7_7. Adv Neurobiol. 2017. PMID: 28353282 Review.
Cited by
-
Leucine Rich Repeat Kinase 2 and Innate Immunity.Front Neurosci. 2020 Mar 10;14:193. doi: 10.3389/fnins.2020.00193. eCollection 2020. Front Neurosci. 2020. PMID: 32210756 Free PMC article. Review.
-
Association of LRRK2 rs11564258 single nucleotide polymorphisms with type and extent of gastrointestinal mycobiome in ulcerative colitis: a case-control study.Gut Pathog. 2021 Sep 30;13(1):56. doi: 10.1186/s13099-021-00453-1. Gut Pathog. 2021. PMID: 34593025 Free PMC article.
-
Immunological Features of LRRK2 Function and Its Role in the Gut-Brain Axis Governing Parkinson's Disease.J Parkinsons Dis. 2023;13(3):279-296. doi: 10.3233/JPD-230021. J Parkinsons Dis. 2023. PMID: 37066923 Free PMC article. Review.
-
Novel Therapeutic Avenues for Hypertrophic Cardiomyopathy.Am J Cardiovasc Drugs. 2023 Nov;23(6):623-640. doi: 10.1007/s40256-023-00609-1. Epub 2023 Sep 5. Am J Cardiovasc Drugs. 2023. PMID: 37670168 Review.
-
Common Neurodegeneration-Associated Proteins Are Physiologically Expressed by Human B Lymphocytes and Are Interconnected via the Inflammation/Autophagy-Related Proteins TRAF6 and SQSTM1.Front Immunol. 2019 Nov 25;10:2704. doi: 10.3389/fimmu.2019.02704. eCollection 2019. Front Immunol. 2019. PMID: 31824497 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

