Micro-pharmacokinetics: Quantifying local drug concentration at live cell membranes
- PMID: 29472588
- PMCID: PMC5823863
- DOI: 10.1038/s41598-018-21100-x
Micro-pharmacokinetics: Quantifying local drug concentration at live cell membranes
Abstract
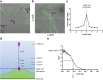
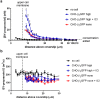
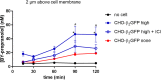
Fundamental equations for determining pharmacological parameters, such as the binding affinity of a ligand for its target receptor, assume a homogeneous distribution of ligand, with concentrations in the immediate vicinity of the receptor being the same as those in the bulk aqueous phase. It is, however, known that drugs are able to interact directly with the plasma membrane, potentially increasing local ligand concentrations around the receptor. We have previously reported an influence of ligand-phospholipid interactions on ligand binding kinetics at the β2-adrenoceptor, which resulted in distinct "micro-pharmacokinetic" ligand profiles. Here, we directly quantified the local concentration of BODIPY630/650-PEG8-S-propranolol (BY-propranolol), a fluorescent derivative of the classical β-blocker propranolol, at various distances above membranes of single living cells using fluorescence correlation spectroscopy. We show for the first time a significantly increased ligand concentration immediately adjacent to the cell membrane compared to the bulk aqueous phase. We further show a clear role of both the cell membrane and the β2-adrenoceptor in determining high local BY-propranolol concentrations at the cell surface. These data suggest that the true binding affinity of BY-propranolol for the β2-adrenoceptor is likely far lower than previously reported and highlights the critical importance of understanding the "micro-pharmacokinetic" profiles of ligands for membrane-associated proteins.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Observed drug-receptor association rates are governed by membrane affinity: the importance of establishing "micro-pharmacokinetic/pharmacodynamic relationships" at the β2-adrenoceptor.Mol Pharmacol. 2014 Apr;85(4):608-17. doi: 10.1124/mol.113.090209. Epub 2014 Jan 29. Mol Pharmacol. 2014. PMID: 24476583
-
G-protein-coupled receptor chromatographic stationary phases. 2. Ligand-induced conformational mobility in an immobilized beta2-adrenergic receptor.Anal Chem. 2004 Dec 15;76(24):7187-93. doi: 10.1021/ac048910c. Anal Chem. 2004. PMID: 15595859
-
Characterization of porcine beta1- and beta2-adrenergic receptors in heart, skeletal muscle, and adipose tissue, and the identification of an atypical beta-adrenergic binding site.J Anim Sci. 2005 Oct;83(10):2339-48. doi: 10.2527/2005.83102339x. J Anim Sci. 2005. PMID: 16160045
-
An approach to analysis of radiolabeled ligand interactions with specific receptors.Eur J Pharmacol. 1999 Dec 15;386(2-3):279-88. doi: 10.1016/s0014-2999(99)00679-2. Eur J Pharmacol. 1999. PMID: 10618480
-
The purification of membranes by affinity partitioning.FASEB J. 1995 Oct;9(13):1304-10. doi: 10.1096/fasebj.9.13.7557020. FASEB J. 1995. PMID: 7557020 Review.
Cited by
-
Pro-phagocytic function and structural basis of GPR84 signaling.Nat Commun. 2023 Sep 14;14(1):5706. doi: 10.1038/s41467-023-41201-0. Nat Commun. 2023. PMID: 37709767 Free PMC article.
-
Pro-phagocytic function and structural basis of GPR84 signaling.Res Sq [Preprint]. 2023 Feb 15:rs.3.rs-2535247. doi: 10.21203/rs.3.rs-2535247/v1. Res Sq. 2023. Update in: Nat Commun. 2023 Sep 14;14(1):5706. doi: 10.1038/s41467-023-41201-0 PMID: 36824923 Free PMC article. Updated. Preprint.
-
Improving ADMET Prediction Accuracy for Candidate Drugs: Factors to Consider in QSPR Modeling Approaches.Curr Top Med Chem. 2024;24(3):222-242. doi: 10.2174/0115680266280005231207105900. Curr Top Med Chem. 2024. PMID: 38083894 Review.
-
Mechanistic Understanding from Molecular Dynamics in Pharmaceutical Research 2: Lipid Membrane in Drug Design.Pharmaceuticals (Basel). 2021 Oct 19;14(10):1062. doi: 10.3390/ph14101062. Pharmaceuticals (Basel). 2021. PMID: 34681286 Free PMC article. Review.
-
Preassembled GPCR signaling complexes mediate distinct cellular responses to ultralow ligand concentrations.Sci Signal. 2018 Oct 9;11(551):eaan1188. doi: 10.1126/scisignal.aan1188. Sci Signal. 2018. PMID: 30301787 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

