Comparison of Disease-Modifying Therapies for the Management of Multiple Sclerosis: Analysis of Healthcare Resource Utilization and Relapse Rates from US Insurance Claims Data
- PMID: 29464673
- PMCID: PMC5820236
- DOI: 10.1007/s41669-017-0035-2
Comparison of Disease-Modifying Therapies for the Management of Multiple Sclerosis: Analysis of Healthcare Resource Utilization and Relapse Rates from US Insurance Claims Data
Abstract
Background: Data on comparative healthcare resource utilization and costs associated with the newer oral disease-modifying therapies (DMTs) for managing relapsing-remitting multiple sclerosis (MS) in routine clinical practice are limited. The purpose of this study was to estimate healthcare resource utilization, costs, and relapse rates in the year after initiating treatment with dimethyl fumarate (DMF), interferon (IFN)-β, glatiramer acetate (GA), teriflunomide, or fingolimod in routine clinical practice for patients with MS who did not receive a DMT in the previous year.
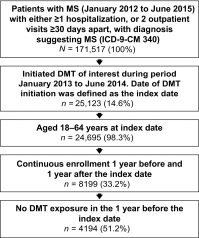
Methods: Patients initiating DMF, IFNβ, GA, teriflunomide, or fingolimod were identified based on claims data from 2012 to 2015 in the Truven MarketScan Commercial Claims Databases (n = 4194). Healthcare resource utilization assessment included the proportion of patients who were hospitalized, or had emergency room (ER) or urgent care (UC) visits. Healthcare costs were estimated for 1 year before and 1 year after DMT initiation. Relapse episodes were identified based on a published claims-based algorithm and clinical input from the research investigators.
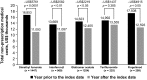
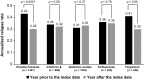
Results: After DMT initiation, significant reductions in the proportions of patients who were hospitalized or requiring ER/UC visits were observed in all patient cohorts (p < 0.001 and p < 0.05, respectively). Non-prescription medical costs decreased after DMT initiation, with the largest decrease observed in the DMF cohort (US$5761 reduction, p < 0.0001). Reductions in non-prescription medical costs were associated with decreased use of outpatient services and inpatient hospital stays, and have the potential to partially offset DMT costs.
Conclusions: DMT initiation is associated with reductions in healthcare resource utilization and non-prescription medical costs in routine clinical practice.
Conflict of interest statement
Conflicts of interest
Jacqueline Nicholas has received research funding from Genzyme, Novartis, Teva, Biogen, and Alexion, and has received consulting and speaking honoraria from Genzyme, Novartis, Teva, Biogen, and Medtronic. Aaron Boster has received research funding from Genentech, Actelion, and Mallinckrodt, and has received consulting and speaking honoraria from Genzyme, Novartis, Teva, Biogen, and Medtronic. Monica Fay and Andrew Lee are employees of, and hold stock and/or stock options in, Biogen. Ning Wu, Wei-Shi Yeh, Jon Kendter, and Ming-Yi Huang were employees of Biogen at the time of the study.
Funding
This study was funded by Biogen (Cambridge, MA, USA).
Ethical approval
This article does not report data from any studies with human participants or animals performed by any of the authors.
Data Availability
The datasets analyzed in the present study are not publicly available because they were licensed with restrictions from a third-party source.
Figures
Similar articles
-
Comparative Effectiveness Research of Disease-Modifying Therapies for the Management of Multiple Sclerosis: Analysis of a Large Health Insurance Claims Database.Neurol Ther. 2017 Jun;6(1):91-102. doi: 10.1007/s40120-017-0064-x. Epub 2017 Feb 16. Neurol Ther. 2017. PMID: 28211024 Free PMC article.
-
Patterns of Utilization and Expenditure Across Multiple Sclerosis Disease-Modifying Therapies: A Retrospective Cohort Study Using Claims Data from a Commercially Insured Population in the United States, 2010-2019.Neurol Ther. 2022 Sep;11(3):1147-1165. doi: 10.1007/s40120-022-00358-4. Epub 2022 May 22. Neurol Ther. 2022. PMID: 35598225 Free PMC article.
-
Comparative effectiveness of dimethyl fumarate versus fingolimod and teriflunomide among MS patients switching from first-generation platform therapies in the US.Mult Scler Relat Disord. 2019 Jan;27:101-111. doi: 10.1016/j.msard.2018.09.038. Epub 2018 Oct 10. Mult Scler Relat Disord. 2019. PMID: 30368221
-
Interferons-beta versus glatiramer acetate for relapsing-remitting multiple sclerosis.Cochrane Database Syst Rev. 2016 Nov 24;11(11):CD009333. doi: 10.1002/14651858.CD009333.pub3. Cochrane Database Syst Rev. 2016. PMID: 27880972 Free PMC article. Review.
-
Teriflunomide for multiple sclerosis.Cochrane Database Syst Rev. 2012 Dec 12;12:CD009882. doi: 10.1002/14651858.CD009882.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2016 Mar 22;3:CD009882. doi: 10.1002/14651858.CD009882.pub3. PMID: 23235682 Updated. Review.
Cited by
-
A comparison of clinical, utilization, and cost outcomes between oral treatments for multiple sclerosis.J Manag Care Spec Pharm. 2024 Feb 3;30(2):129-140. doi: 10.18553/jmcp.2024.30.2.129. J Manag Care Spec Pharm. 2024. PMID: 38308623 Free PMC article.
-
Economic burden of multiple sclerosis in the United States: A systematic literature review.J Manag Care Spec Pharm. 2023 Dec;29(12):1354-1368. doi: 10.18553/jmcp.2023.23039. Epub 2023 Nov 17. J Manag Care Spec Pharm. 2023. PMID: 37976077 Free PMC article. Review.
-
Assessing the Health Economic Outcomes from Commercially Insured Relapsing Multiple Sclerosis Patients Who Switched from Other Disease-Modifying Therapies to Teriflunomide, in the United States.Clinicoecon Outcomes Res. 2023 May 20;15:361-373. doi: 10.2147/CEOR.S401687. eCollection 2023. Clinicoecon Outcomes Res. 2023. PMID: 37234086 Free PMC article.
-
Health economic outcomes of switching to alemtuzumab from other disease-modifying therapies in people with multiple sclerosis in the USA.J Comp Eff Res. 2023 Jan;12(1):e220127. doi: 10.2217/cer-2022-0127. Epub 2022 Nov 28. J Comp Eff Res. 2023. PMID: 36440609 Free PMC article.
-
Factors associated with oral fingolimod use over injectable disease- modifying agent use in multiple sclerosis.Explor Res Clin Soc Pharm. 2021 May 5;2:100021. doi: 10.1016/j.rcsop.2021.100021. eCollection 2021 Jun. Explor Res Clin Soc Pharm. 2021. PMID: 35481133 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

