Inhibition of non-homologous end joining in Fanconi Anemia cells results in rescue of survival after interstrand crosslinks but sensitization to replication associated double-strand breaks
- PMID: 29459202
- PMCID: PMC6054796
- DOI: 10.1016/j.dnarep.2018.02.003
Inhibition of non-homologous end joining in Fanconi Anemia cells results in rescue of survival after interstrand crosslinks but sensitization to replication associated double-strand breaks
Abstract
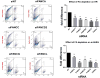
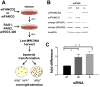
When Fanconi Anemia (FA) proteins were depleted in human U2OS cells with integrated DNA repair reporters, we observed decreases in homologous recombination (HR), decreases in mutagenic non-homologous end joining (m-NHEJ) and increases in canonical NHEJ, which was independently confirmed by measuring V(D)J recombination. Furthermore, depletion of FA proteins resulted in reduced HR protein foci and increased NHEJ protein recruitment to replication-associated DSBs, consistent with our observation that the use of canonical NHEJ increases after depletion of FA proteins in cycling cells. FA-depleted cells and FA-mutant cells were exquisitely sensitive to a DNA-PKcs inhibitor (DNA-PKi) after sustaining replication-associated double strand breaks (DSBs). By contrast, after DNA interstrand crosslinks, DNA-PKi resulted in increased survival in FA-deficient cells, implying that NHEJ is contributing to lethality after crosslink repair. Our results suggest FA proteins inhibit NHEJ, since repair intermediates from crosslinks are rendered lethal by NHEJ. The implication is that bone marrow failure in FA could be triggered by naturally occurring DNA crosslinks, and DNA-PK inhibitors would be protective. Since some sporadic cancers have been shown to have deficiencies in the FA-pathway, these tumors should be vulnerable to NHEJ inhibitors with replication stress, but not with crosslinking agents, which could be tested in future clinical trials.
Keywords: Double strand break repair; Fanconi anemia; Homologous recombination; Interstrand crosslinks; Non-homologous end joining.
Copyright © 2018 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures

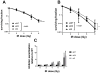


Similar articles
-
Compromised repair of radiation-induced DNA double-strand breaks in Fanconi anemia fibroblasts in G2.DNA Repair (Amst). 2020 Dec;96:102992. doi: 10.1016/j.dnarep.2020.102992. Epub 2020 Oct 6. DNA Repair (Amst). 2020. PMID: 33069004
-
The non-homologous end-joining activity is required for Fanconi anemia fetal HSC maintenance.Stem Cell Res Ther. 2019 Mar 29;10(1):114. doi: 10.1186/s13287-019-1206-0. Stem Cell Res Ther. 2019. PMID: 30925933 Free PMC article.
-
Hyper-active non-homologous end joining selects for synthetic lethality resistant and pathological Fanconi anemia hematopoietic stem and progenitor cells.Sci Rep. 2016 Feb 26;6:22167. doi: 10.1038/srep22167. Sci Rep. 2016. PMID: 26916217 Free PMC article.
-
Beyond interstrand crosslinks repair: contribution of FANCD2 and other Fanconi Anemia proteins to the replication of DNA.Mutat Res. 2018 Mar;808:83-92. doi: 10.1016/j.mrfmmm.2017.09.004. Epub 2017 Sep 14. Mutat Res. 2018. PMID: 29031493 Review.
-
FANCJ helicase operates in the Fanconi Anemia DNA repair pathway and the response to replicational stress.Curr Mol Med. 2009 May;9(4):470-82. doi: 10.2174/156652409788167159. Curr Mol Med. 2009. PMID: 19519404 Free PMC article. Review.
Cited by
-
Type-I Interferon Signaling in Fanconi Anemia.Front Cell Infect Microbiol. 2022 Feb 7;12:820273. doi: 10.3389/fcimb.2022.820273. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35198459 Free PMC article. Review.
-
Cooperation of the ATM and Fanconi Anemia/BRCA Pathways in Double-Strand Break End Resection.Cell Rep. 2020 Feb 18;30(7):2402-2415.e5. doi: 10.1016/j.celrep.2020.01.052. Cell Rep. 2020. PMID: 32075772 Free PMC article.
-
Disabling the Fanconi Anemia Pathway in Stem Cells Leads to Radioresistance and Genomic Instability.Cancer Res. 2021 Jul 1;81(13):3706-3716. doi: 10.1158/0008-5472.CAN-20-3309. Epub 2021 May 3. Cancer Res. 2021. PMID: 33941615 Free PMC article.
-
Beta Human Papillomavirus 8E6 Attenuates Non-Homologous End Joining by Hindering DNA-PKcs Activity.Cancers (Basel). 2020 Aug 20;12(9):2356. doi: 10.3390/cancers12092356. Cancers (Basel). 2020. PMID: 32825402 Free PMC article.
-
DNMT3A Harboring Leukemia-Associated Mutations Directs Sensitivity to DNA Damage at Replication Forks.Clin Cancer Res. 2022 Feb 15;28(4):756-769. doi: 10.1158/1078-0432.CCR-21-2863. Clin Cancer Res. 2022. PMID: 34716195 Free PMC article.
References
-
- Deriano L, Roth DB. Modernizing the nonhomologous end-joining repertoire: alternative and classical NHEJ share the stage. Annual review of genetics. 2013;47:433–455. - PubMed
-
- Alter BP. Fanconi’s anemia and malignancies. American journal of hematology. 1996;53:99–110. - PubMed
-
- Fanconi G. Familial constitutional panmyelocytopathy, Fanconi’s anemia (F.A.). I. Clinical aspects. Seminars in hematology. 1967;4:233–240. - PubMed
-
- Schroeder TM, Tilgen D, Kruger J, Vogel F. Formal genetics of Fanconi’s anemia. Human genetics. 1976;32:257–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

