Snail regulates BMP and TGFβ pathways to control the differentiation status of glioma-initiating cells
- PMID: 29449696
- PMCID: PMC5945579
- DOI: 10.1038/s41388-018-0136-0
Snail regulates BMP and TGFβ pathways to control the differentiation status of glioma-initiating cells
Abstract
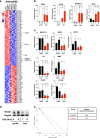
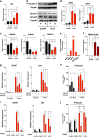
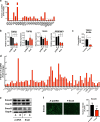
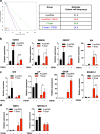
Glioblastoma multiforme is a brain malignancy characterized by high heterogeneity, invasiveness, and resistance to current therapies, attributes related to the occurrence of glioma stem cells (GSCs). Transforming growth factor β (TGFβ) promotes self-renewal and bone morphogenetic protein (BMP) induces differentiation of GSCs. BMP7 induces the transcription factor Snail to promote astrocytic differentiation in GSCs and suppress tumor growth in vivo. We demonstrate that Snail represses stemness in GSCs. Snail interacts with SMAD signaling mediators, generates a positive feedback loop of BMP signaling and transcriptionally represses the TGFB1 gene, decreasing TGFβ1 signaling activity. Exogenous TGFβ1 counteracts Snail function in vitro, and in vivo promotes proliferation and re-expression of Nestin, confirming the importance of TGFB1 gene repression by Snail. In conclusion, novel insight highlights mechanisms whereby Snail differentially regulates the activity of the opposing BMP and TGFβ pathways, thus promoting an astrocytic fate switch and repressing stemness in GSCs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Snail depletes the tumorigenic potential of glioblastoma.Oncogene. 2013 Nov 21;32(47):5409-20. doi: 10.1038/onc.2013.67. Epub 2013 Mar 25. Oncogene. 2013. PMID: 23524585 Free PMC article.
-
BMP signaling mediates glioma stem cell quiescence and confers treatment resistance in glioblastoma.Sci Rep. 2019 Oct 10;9(1):14569. doi: 10.1038/s41598-019-51270-1. Sci Rep. 2019. PMID: 31602000 Free PMC article.
-
HDAC6 inhibition induces glioma stem cells differentiation and enhances cellular radiation sensitivity through the SHH/Gli1 signaling pathway.Cancer Lett. 2018 Feb 28;415:164-176. doi: 10.1016/j.canlet.2017.12.005. Epub 2017 Dec 6. Cancer Lett. 2018. PMID: 29222038
-
Overview of Transforming Growth Factor β Superfamily Involvement in Glioblastoma Initiation and Progression.Asian Pac J Cancer Prev. 2015;16(16):6813-23. doi: 10.7314/apjcp.2015.16.16.6813. Asian Pac J Cancer Prev. 2015. PMID: 26514451 Review.
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
Cited by
-
The TGF-β Family in Glioblastoma.Int J Mol Sci. 2024 Jan 15;25(2):1067. doi: 10.3390/ijms25021067. Int J Mol Sci. 2024. PMID: 38256140 Free PMC article. Review.
-
Competition between type I activin and BMP receptors for binding to ACVR2A regulates signaling to distinct Smad pathways.BMC Biol. 2022 Feb 18;20(1):50. doi: 10.1186/s12915-022-01252-z. BMC Biol. 2022. PMID: 35177083 Free PMC article.
-
Targeting chemoresistant colorectal cancer via systemic administration of a BMP7 variant.Oncogene. 2020 Jan;39(5):987-1003. doi: 10.1038/s41388-019-1047-4. Epub 2019 Oct 7. Oncogene. 2020. PMID: 31591478 Free PMC article.
-
Bone Morphogenetic Protein Signaling in Cancer; Some Topics in the Recent 10 Years.Front Cell Dev Biol. 2022 May 25;10:883523. doi: 10.3389/fcell.2022.883523. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35693928 Free PMC article. Review.
-
Bone marrow mesenchymal stem cell-derived exosomal miR-34c-5p ameliorates RIF by inhibiting the core fucosylation of multiple proteins.Mol Ther. 2022 Feb 2;30(2):763-781. doi: 10.1016/j.ymthe.2021.10.012. Epub 2021 Oct 19. Mol Ther. 2022. PMID: 34678513 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

