Cinacalcet versus Placebo for secondary hyperparathyroidism in chronic kidney disease patients: a meta-analysis of randomized controlled trials and trial sequential analysis
- PMID: 29449603
- PMCID: PMC5814442
- DOI: 10.1038/s41598-018-21397-8
Cinacalcet versus Placebo for secondary hyperparathyroidism in chronic kidney disease patients: a meta-analysis of randomized controlled trials and trial sequential analysis
Abstract
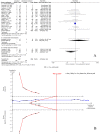
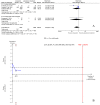
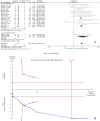
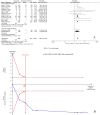
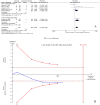
To assess the efficacy and safety of cinacalcet on secondary hyperparathyroidism in patients with chronic kidney disease, Pubmed, Embase, and the Cochrane Central Register of Controlled Trials were searched until March 2016. Trial sequential analysis (TSA) was conducted to control the risks of type I and II errors and calculate required information size (RIS). A total of 25 articles with 8481 participants were included. Compared with controls, cinacalcet administration did not reduce all-cause mortality (RR = 0.97, 95% CI = 0.89-1.05, P = 0.41, TSA-adjusted 95% CI = 0.86-1.08, RIS = 5260, n = 8386) or cardiovascular mortality (RR = 0.95, 95% CI = 0.83-1.07, P = 0.39, TSA-adjusted 95% CI = 0.70-1.26, RIS = 3780 n = 5418), but it reduced the incidence of parathyroidectomy (RR = 0.48, 95% CI = 0.40-0.50, P < 0.001, TSA-adjusted 95% CI = 0.39-0.60, RIS = 5787 n = 5488). Cinacalcet increased the risk of hypocalcemia (RR = 8.48, 95% CI = 6.37-11.29, P < 0.001, TSA-adjusted 95% CI = 5.25-13.70, RIS = 6522, n = 7785), nausea (RR = 2.12, 95% CI = 1.62-2.77, P < 0.001, TSA-adjusted 95% CI = 1.45-3.04, RIS = 4684, n = 7512), vomiting (RR = 2.00, 95% CI = 1.79-2.24, P < 0.001, TSA-adjusted 95% CI = 1.77-2.26, RIS = 1374, n = 7331) and diarrhea (RR = 1.17, 95% CI = 1.05-1.32, P = 0.006, TSA-adjusted 95% CI = 1.02-1.36, RIS = 8388, n = 6116). Cinacalcet did not significantly reduce the incidence of fractures (RR = 0.58, 95% CI = 0.21-1.59, P = 0.29, TSA-adjusted 95% CI = 0.01-35.11, RIS = 76376, n = 4053). Cinacalcet reduced the incidence of parathyroidectomy, however, it did not reduce all-cause and cardiovascular mortality, and increased the risk of adverse events including hypocalcemia and gastrointestinal disorders.
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Cinacalcet plus vitamin D versus vitamin D alone for the treatment of secondary hyperparathyroidism in patients undergoing dialysis: a meta-analysis of randomized controlled trials.Int Urol Nephrol. 2019 Nov;51(11):2027-2036. doi: 10.1007/s11255-019-02271-6. Epub 2019 Sep 17. Int Urol Nephrol. 2019. PMID: 31531805
-
Comparative Effectiveness of Calcimimetic Agents for Secondary Hyperparathyroidism in Adults: A Systematic Review and Network Meta-analysis.Am J Kidney Dis. 2020 Sep;76(3):321-330. doi: 10.1053/j.ajkd.2020.02.439. Epub 2020 May 28. Am J Kidney Dis. 2020. PMID: 32475604
-
Efficacy and safety of cinacalcet compared with other treatments for secondary hyperparathyroidism in patients with chronic kidney disease or end-stage renal disease: a meta-analysis.BMC Nephrol. 2020 Jul 31;21(1):316. doi: 10.1186/s12882-019-1639-9. BMC Nephrol. 2020. PMID: 32736534 Free PMC article.
-
Cinacalcet versus standard treatment for chronic kidney disease: a systematic review and meta-analysis.Ren Fail. 2016 Jul;38(6):857-74. doi: 10.3109/0886022X.2016.1172468. Epub 2016 May 2. Ren Fail. 2016. PMID: 27137817 Review.
-
Cinacalcet in patients with chronic kidney disease: a cumulative meta-analysis of randomized controlled trials.PLoS Med. 2013;10(4):e1001436. doi: 10.1371/journal.pmed.1001436. Epub 2013 Apr 30. PLoS Med. 2013. PMID: 23637579 Free PMC article. Review.
Cited by
-
Cinacalcet attenuated bone loss via inhibiting parathyroid hormone-induced endothelial-to-adipocyte transition in chronic kidney disease rats.Ann Transl Med. 2019 Jul;7(14):312. doi: 10.21037/atm.2019.06.44. Ann Transl Med. 2019. PMID: 31475182 Free PMC article.
-
Cinacalcet may suppress kidney enlargement in hemodialysis patients with autosomal dominant polycystic kidney disease.Sci Rep. 2021 May 11;11(1):10014. doi: 10.1038/s41598-021-89480-1. Sci Rep. 2021. PMID: 33976330 Free PMC article.
-
Cinacalcet plus vitamin D versus vitamin D alone for the treatment of secondary hyperparathyroidism in patients undergoing dialysis: a meta-analysis of randomized controlled trials.Int Urol Nephrol. 2019 Nov;51(11):2027-2036. doi: 10.1007/s11255-019-02271-6. Epub 2019 Sep 17. Int Urol Nephrol. 2019. PMID: 31531805
-
Treatment of hyperphosphatemia: the dangers of high PTH levels.Pediatr Nephrol. 2020 Mar;35(3):493-500. doi: 10.1007/s00467-019-04400-w. Epub 2019 Nov 6. Pediatr Nephrol. 2020. PMID: 31696357 Review.
-
Effects of active vitamin D analogs and calcimimetic agents on PTH and bone mineral biomarkers in hemodialysis patients with SHPT: a network meta-analysis.Eur J Clin Pharmacol. 2024 Oct;80(10):1555-1569. doi: 10.1007/s00228-024-03730-5. Epub 2024 Jul 13. Eur J Clin Pharmacol. 2024. PMID: 39002024
References
-
- Mei, C. et al. Efficacy and safety of Cinacalcet on secondary hyperparathyroidism in Chinese chronic kidney disease patients receiving hemodialysis. Hemodialysis international. International Symposium on Home Hemodialysis, 10.1111/hdi.12410 (2016). - PubMed
-
- Marco, M. P. et al. Higher impact of mineral metabolism on cardiovascular mortality in a European hemodialysis population. Kidney international. Supplement, S111–114, 10.1046/j.1523-1755.63.s85.26.x (2003). - PubMed
-
- Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. American journal of kidney diseases: the official journal of the National Kidney Foundation. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

