Long non-coding RNA PVT1 predicts poor prognosis and induces radioresistance by regulating DNA repair and cell apoptosis in nasopharyngeal carcinoma
- PMID: 29445147
- PMCID: PMC5833381
- DOI: 10.1038/s41419-018-0265-y
Long non-coding RNA PVT1 predicts poor prognosis and induces radioresistance by regulating DNA repair and cell apoptosis in nasopharyngeal carcinoma
Abstract
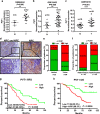
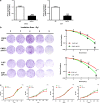
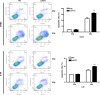
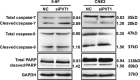
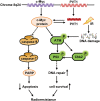
The long non-coding RNA, plasmacytoma variant translocation 1 (PVT1), is highly expressed in a variety of tumors, and is believed to be a potential oncogene. However, the role and mechanism of action of PVT1 in the carcinogenesis and progression of nasopharyngeal carcinomas (NPCs) remains unclear. In this study, for the first time, we have discovered that PVT1 shows higher expression in NPCs than in normal nasopharyngeal epithelial tissue, and patients with NPCs who show higher expression of PVT1 have worse progression-free and overall survivals. Additionally, we observed that the proliferation of NPC cells decreased, and their rate of apoptosis increased; these results indicated that the knockdown of PVT1 expression in the NPC cells induced radiosensitivity. Further, we have shown that the knockdown of PVT1 expression can induce apoptosis in the NPC cells by influencing the DNA damage repair pathway after radiotherapy. In general, our study shows that PVT1 may be a novel biomarker for prognosis and a new target for the treatment of NPCs. Additionally, targeting PVT1 may be a potential strategy for the clinical management of NPC and for the improvement of the curative effect of radiation in NPCs.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
RPA3 is a potential marker of prognosis and radioresistance for nasopharyngeal carcinoma.J Cell Mol Med. 2017 Nov;21(11):2872-2883. doi: 10.1111/jcmm.13200. Epub 2017 May 30. J Cell Mol Med. 2017. PMID: 28557284 Free PMC article.
-
lncRNA CASC19 Contributes to Radioresistance of Nasopharyngeal Carcinoma by Promoting Autophagy via AMPK-mTOR Pathway.Int J Mol Sci. 2021 Jan 30;22(3):1407. doi: 10.3390/ijms22031407. Int J Mol Sci. 2021. PMID: 33573349 Free PMC article.
-
RPA1 downregulation enhances nasopharyngeal cancer radiosensitivity via blocking RAD51 to the DNA damage site.Exp Cell Res. 2018 Oct 15;371(2):330-341. doi: 10.1016/j.yexcr.2018.08.025. Epub 2018 Aug 23. Exp Cell Res. 2018. PMID: 30144445
-
Long non-coding RNA PVT1 interacts with MYC and its downstream molecules to synergistically promote tumorigenesis.Cell Mol Life Sci. 2019 Nov;76(21):4275-4289. doi: 10.1007/s00018-019-03222-1. Epub 2019 Jul 15. Cell Mol Life Sci. 2019. PMID: 31309249 Free PMC article. Review.
-
Prognostic values of long noncoding RNA PVT1 in various carcinomas: An updated systematic review and meta-analysis.Cell Prolif. 2018 Dec;51(6):e12519. doi: 10.1111/cpr.12519. Epub 2018 Sep 5. Cell Prolif. 2018. PMID: 30252166 Free PMC article. Review.
Cited by
-
LncRNA HOTAIRM1 promotes radioresistance in nasopharyngeal carcinoma by modulating FTO acetylation-dependent alternative splicing of CD44.Neoplasia. 2024 Oct;56:101034. doi: 10.1016/j.neo.2024.101034. Epub 2024 Aug 10. Neoplasia. 2024. PMID: 39128424 Free PMC article.
-
CircCDYL2 bolsters radiotherapy resistance in nasopharyngeal carcinoma by promoting RAD51 translation initiation for enhanced homologous recombination repair.J Exp Clin Cancer Res. 2024 Apr 23;43(1):122. doi: 10.1186/s13046-024-03049-0. J Exp Clin Cancer Res. 2024. PMID: 38654320 Free PMC article.
-
Role of long non-coding RNA in chemoradiotherapy resistance of nasopharyngeal carcinoma.Front Oncol. 2024 Feb 29;14:1346413. doi: 10.3389/fonc.2024.1346413. eCollection 2024. Front Oncol. 2024. PMID: 38487724 Free PMC article. Review.
-
PVT1 promotes proliferation and macrophage immunosuppressive polarization through STAT1 and CX3CL1 regulation in glioblastoma multiforme.CNS Neurosci Ther. 2024 Jan;30(1):e14566. doi: 10.1111/cns.14566. CNS Neurosci Ther. 2024. PMID: 38287522 Free PMC article.
-
lncRNAs are potential prognostic markers in patients with nasopharyngeal carcinoma in China: A systematic review and meta‑analysis.Mol Clin Oncol. 2023 Dec 14;20(2):11. doi: 10.3892/mco.2023.2709. eCollection 2024 Feb. Mol Clin Oncol. 2023. PMID: 38213659 Free PMC article.
References
-
- Thompson L. Nasopharyngeal carcinoma. Ear. Nose. Throat J. 2005;84:404–405. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

