Sex bias in MHC I-associated shaping of the adaptive immune system
- PMID: 29440397
- PMCID: PMC5834686
- DOI: 10.1073/pnas.1716146115
Sex bias in MHC I-associated shaping of the adaptive immune system
Abstract
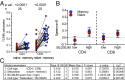
HLA associations, T cell receptor (TCR) repertoire bias, and sex bias have independently been shown for many diseases. While some immunological differences between the sexes have been described, they do not fully explain bias in men toward many infections/cancers, and toward women in autoimmunity. Next-generation TCR variable beta chain (TCRBV) immunosequencing of 824 individuals was evaluated in a multiparametric analysis including HLA-A -B/MHC class I background, TCRBV usage, sex, age, ethnicity, and TCRBV selection/expansion dynamics. We found that HLA-associated shaping of TCRBV usage differed between the sexes. Furthermore, certain TCRBVs were selected and expanded in unison. Correlations between these TCRBV relationships and biochemical similarities in HLA-binding positions were different in CD8 T cells of patients with autoimmune diseases (multiple sclerosis and rheumatoid arthritis) compared with healthy controls. Within patients, men showed higher TCRBV relationship Spearman's rhos in relation to HLA-binding position similarities compared with women. In line with this, CD8 T cells of men with autoimmune diseases also showed higher degrees of TCRBV perturbation compared with women. Concerted selection and expansion of CD8 T cells in patients with autoimmune diseases, but especially in men, appears to be less dependent on high HLA-binding similarity than in CD4 T cells. These findings are consistent with studies attributing autoimmunity to processes of epitope spreading and expansion of low-avidity T cell clones and may have further implications for the interpretation of pathogenic mechanisms of infectious and autoimmune diseases with known HLA associations. Reanalysis of some HLA association studies, separating the data by sex, could be informative.
Keywords: T cell receptor repertoire; autoimmunity; immunological sex bias; multiple sclerosis; rheumatoid arthritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
TCR beta-chain variable region-driven selection and massive expansion of HLA-class I-restricted antitumor CTL lines from HLA-A*0201+ melanoma patients.J Immunol. 1997 Jun 15;158(12):5902-13. J Immunol. 1997. PMID: 9190943
-
Genetic influence on the shaping of the human T-cell receptor repertoire: quantitative assessment by competitive polymerase chain reaction.Scand J Immunol. 1996 Aug;44(2):173-8. doi: 10.1046/j.1365-3083.1996.d01-286.x. Scand J Immunol. 1996. PMID: 8711431
-
Altered Repertoire Diversity and Disease-Associated Clonal Expansions Revealed by T Cell Receptor Immunosequencing in Ankylosing Spondylitis Patients.Arthritis Rheumatol. 2020 Aug;72(8):1289-1302. doi: 10.1002/art.41252. Epub 2020 Jul 13. Arthritis Rheumatol. 2020. PMID: 32162785
-
Identification of autoantigen epitopes in MHC class II transgenic mice.Immunol Rev. 1998 Aug;164:129-38. doi: 10.1111/j.1600-065x.1998.tb01215.x. Immunol Rev. 1998. PMID: 9795771 Review.
-
How the immune system achieves self-nonself discrimination during adaptive immunity.Adv Immunol. 2009;102:95-133. doi: 10.1016/S0065-2776(09)01202-4. Adv Immunol. 2009. PMID: 19477320 Review.
Cited by
-
Next-Generation HLA Sequence Analysis Uncovers Shared Risk Alleles Between Clinically Distinct Forms of Childhood Uveitis.Invest Ophthalmol Vis Sci. 2021 Jul 1;62(9):19. doi: 10.1167/iovs.62.9.19. Invest Ophthalmol Vis Sci. 2021. PMID: 34254975 Free PMC article.
-
Evidence for sexual conflict over major histocompatibility complex diversity in a wild songbird.Proc Biol Sci. 2018 Aug 1;285(1884):20180841. doi: 10.1098/rspb.2018.0841. Proc Biol Sci. 2018. PMID: 30068671 Free PMC article.
-
Germline HLA-B evolutionary divergence influences the efficacy of immune checkpoint blockade therapy in gastrointestinal cancer.Genome Med. 2021 Nov 3;13(1):175. doi: 10.1186/s13073-021-00997-6. Genome Med. 2021. PMID: 34732240 Free PMC article.
-
Reference-based comparison of adaptive immune receptor repertoires.Cell Rep Methods. 2022 Aug 22;2(8):100269. doi: 10.1016/j.crmeth.2022.100269. eCollection 2022 Aug 22. Cell Rep Methods. 2022. PMID: 36046619 Free PMC article.
-
Strength of immune selection in tumors varies with sex and age.Nat Commun. 2020 Aug 17;11(1):4128. doi: 10.1038/s41467-020-17981-0. Nat Commun. 2020. PMID: 32807809 Free PMC article.
References
-
- Kappler J, et al. The major histocompatibility complex-restricted antigen receptor on T cells in mouse and man: Identification of constant and variable peptides. Cell. 1983;35:295–302. - PubMed
-
- Arstila TP, et al. A direct estimate of the human alphabeta T cell receptor diversity. Science. 1999;286:958–961. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

