Trovafloxacin attenuates neuroinflammation and improves outcome after traumatic brain injury in mice
- PMID: 29439712
- PMCID: PMC5812039
- DOI: 10.1186/s12974-018-1069-9
Trovafloxacin attenuates neuroinflammation and improves outcome after traumatic brain injury in mice
Abstract
Background: Trovafloxacin is a broad-spectrum antibiotic, recently identified as an inhibitor of pannexin-1 (Panx1) channels. Panx1 channels are important conduits for the adenosine triphosphate (ATP) release from live and dying cells that enhances the inflammatory response of immune cells. Elevated extracellular levels ATP released upon injury activate purinergic pathways in inflammatory cells that promote migration, proliferation, phagocytosis, and apoptotic signals. Here, we tested whether trovafloxacin administration attenuates the neuroinflammatory response and improves outcomes after brain trauma.
Methods: The murine controlled cortical impact (CCI) model was used to determine whether in vivo delivery of trovafloxacin has anti-inflammatory and neuroprotective actions after brain trauma. Locomotor deficit was assessed using the rotarod test. Levels of tissue damage markers and inflammation were measured using western blot, qPCR, and immunofluorescence. In vitro assays were used to evaluate whether trovafloxacin blocks ATP release and cell migration in a chemotactic-stimulated microglia cell line.
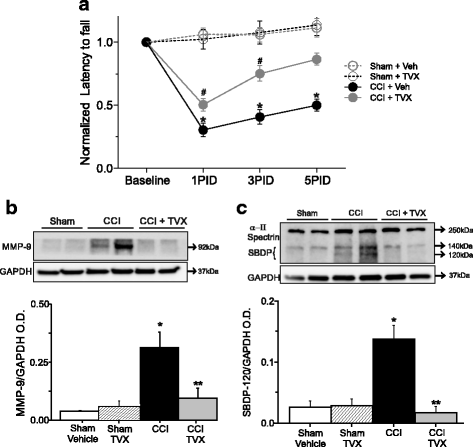
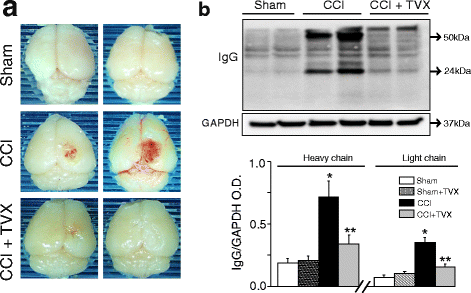
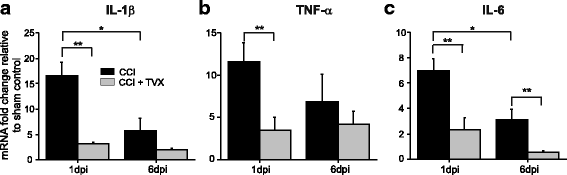
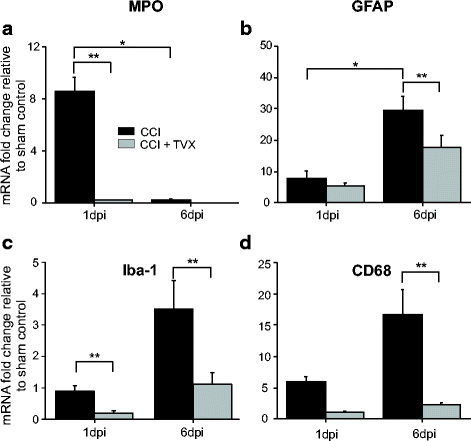
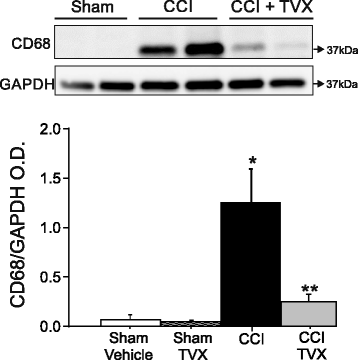
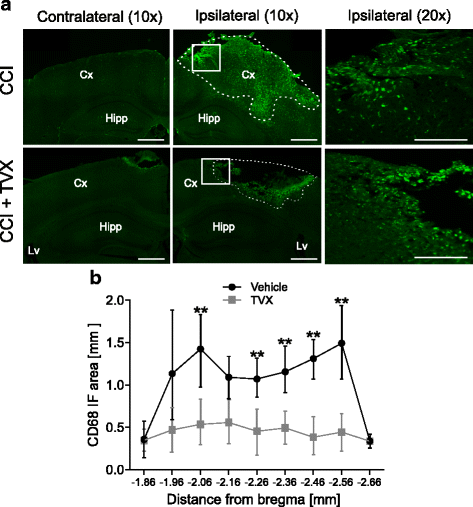
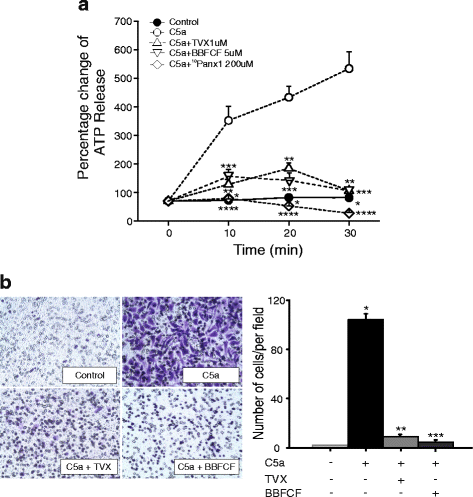
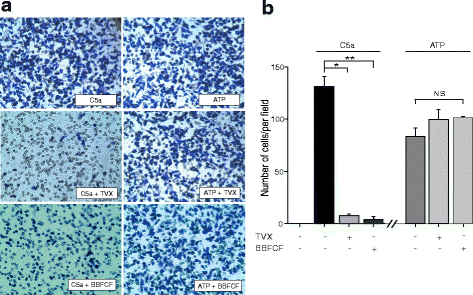
Results: Trovafloxacin treatment of CCI-injured mice significantly reduced tissue damage markers and improved locomotor deficits. In addition, trovafloxacin treatment significantly reduced mRNA levels of several pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α), which correlates with an overall reduction in the accumulation of inflammatory cell types (neutrophils, microglia/macrophages, and astroglia) at the injury zone. To determine whether trovafloxacin exerted these effects by direct action on immune cells, we evaluated its effect on ATP release and cell migration using a chemotactic-stimulated microglial cell line. We found that trovafloxacin significantly inhibited both ATP release and migration of these cells.
Conclusion: Our results show that trovafloxacin administration has pronounced anti-inflammatory and neuroprotective effects following brain injury. These findings lay the foundation for future studies to directly test a role for Panx1 channels in pathological inflammation following brain trauma.
Keywords: Brain injury; Hemichannel; Microglia; Neuroinflammation; Pannexin.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Myeloid Pannexin-1 mediates acute leukocyte infiltration and leads to worse outcomes after brain trauma.J Neuroinflammation. 2020 Aug 20;17(1):245. doi: 10.1186/s12974-020-01917-y. J Neuroinflammation. 2020. PMID: 32819386 Free PMC article.
-
Anti-inflammatory and immunomodulatory mechanisms of atorvastatin in a murine model of traumatic brain injury.J Neuroinflammation. 2017 Aug 23;14(1):167. doi: 10.1186/s12974-017-0934-2. J Neuroinflammation. 2017. PMID: 28835272 Free PMC article.
-
Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury.J Neuroinflammation. 2017 Mar 15;14(1):47. doi: 10.1186/s12974-017-0819-4. J Neuroinflammation. 2017. PMID: 28292310 Free PMC article.
-
Pannexin-1 Channels as Mediators of Neuroinflammation.Int J Mol Sci. 2021 May 14;22(10):5189. doi: 10.3390/ijms22105189. Int J Mol Sci. 2021. PMID: 34068881 Free PMC article. Review.
-
Neuronal injury in chronic CNS inflammation.Best Pract Res Clin Anaesthesiol. 2010 Dec;24(4):551-62. doi: 10.1016/j.bpa.2010.11.001. Epub 2010 Nov 29. Best Pract Res Clin Anaesthesiol. 2010. PMID: 21619866 Review.
Cited by
-
Current state of neuroprotective therapy using antibiotics in human traumatic brain injury and animal models.BMC Neurosci. 2024 Feb 29;25(1):10. doi: 10.1186/s12868-024-00851-6. BMC Neurosci. 2024. PMID: 38424488 Free PMC article. Review.
-
Leveraging the ATP-P2X7 receptor signalling axis to alleviate traumatic CNS damage and related complications.Med Res Rev. 2023 Sep;43(5):1346-1373. doi: 10.1002/med.21952. Epub 2023 Mar 16. Med Res Rev. 2023. PMID: 36924449 Free PMC article. Review.
-
Novel Insights into Parkin-Mediated Mitochondrial Dysfunction and "Mito-Inflammation" in α-Synuclein Toxicity. The Role of the cGAS-STING Signalling Pathway.J Inflamm Res. 2024 Jul 11;17:4549-4574. doi: 10.2147/JIR.S468609. eCollection 2024. J Inflamm Res. 2024. PMID: 39011416 Free PMC article. Review.
-
Pannexin1: insight into inflammatory conditions and its potential involvement in multiple organ dysfunction syndrome.Front Immunol. 2023 Aug 30;14:1217366. doi: 10.3389/fimmu.2023.1217366. eCollection 2023. Front Immunol. 2023. PMID: 37711629 Free PMC article. Review.
-
Regulators of phagocytosis as pharmacologic targets for stroke treatment.Front Pharmacol. 2023 Aug 2;14:1122527. doi: 10.3389/fphar.2023.1122527. eCollection 2023. Front Pharmacol. 2023. PMID: 37601043 Free PMC article. Review.
References
-
- Gootz TD, Zaniewski R, Haskell S, Schmieder B, Tankovic J, Girard D, Courvalin P, Polzer RJ. Activity of the new fluoroquinolone trovafloxacin (CP-99,219) against DNA gyrase and topoisomerase IV mutants of Streptococcus pneumoniae selected in vitro. Antimicrob Agents Chemother. 1996;40:2691–2697. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

