α1-Antitrypsin infusion for treatment of steroid-resistant acute graft-versus-host disease
- PMID: 29437593
- PMCID: PMC5865235
- DOI: 10.1182/blood-2017-11-815746
α1-Antitrypsin infusion for treatment of steroid-resistant acute graft-versus-host disease
Abstract
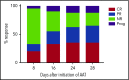
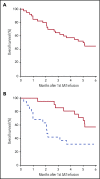
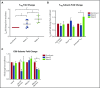
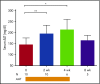
Corticosteroid resistance after acute graft-versus-host disease (SR-aGVHD) results in high morbidity and mortality after allogeneic hematopoietic cell transplantation. Current immunosuppressive therapies for SR-aGVHD provide marginal effectiveness because of poor response or excessive toxicity, primarily from infection. α1-Antitrypsin (AAT), a naturally abundant serine protease inhibitor, is capable of suppressing experimental GVHD by downmodulating inflammation and increasing ratios of regulatory (Treg) to effector T cells (Teffs). In this prospective multicenter clinical study, we sought to determine the safety and response rate of AAT administration in SR-aGVHD. Forty patients with a median age of 59 years received intravenous AAT twice weekly for 4 weeks as first-line treatment of SR-aGVHD. The primary end point was overall response rate (ORR), the proportion of patients with SR-aGVHD in complete (CR) or partial response by day 28 without addition of further immunosuppression. Treatment was well tolerated without drug-related adverse events. A significant increase in serum levels of AAT was observed after treatment. The ORR and CR rates by day 28 were 65% and 35%, respectively, and included responses in all aGVHD target organs. At day 60, responses were sustained in 73% of patients without intervening immunosuppression. Infectious mortality was 10% at 6 months and 2.5% within 30 days of last AAT infusion. Consistent with preclinical data, correlative samples showed an increase in ratio of activated Tregs to Teffs after AAT treatment. These data suggest that AAT is safe and may be potentially efficacious in treating SR-aGVHD. This trial was registered at www.clinicaltrials.gov as #NCT01700036.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
Similar articles
-
Human-Derived α1-Antitrypsin is Still Efficacious in Heavily Pretreated Patients with Steroid-Resistant Gastrointestinal Graft-versus-Host Disease.Biol Blood Marrow Transplant. 2020 Sep;26(9):1620-1626. doi: 10.1016/j.bbmt.2020.05.014. Epub 2020 May 25. Biol Blood Marrow Transplant. 2020. PMID: 32454215
-
A Prospective Study of Alemtuzumab as a Second-Line Agent for Steroid-Refractory Acute Graft-versus-Host Disease in Pediatric and Young Adult Allogeneic Hematopoietic Stem Cell Transplantation.Biol Blood Marrow Transplant. 2016 Dec;22(12):2220-2225. doi: 10.1016/j.bbmt.2016.09.016. Epub 2016 Sep 21. Biol Blood Marrow Transplant. 2016. PMID: 27664325 Free PMC article.
-
Alpha-1-Antitrypsin Experience for Steroid-Resistant Acute Graft-Versus-Host Disease.Indian J Hematol Blood Transfus. 2022 Jul;38(3):601-605. doi: 10.1007/s12288-022-01524-2. Epub 2022 Feb 7. Indian J Hematol Blood Transfus. 2022. PMID: 35747565 Free PMC article.
-
Response of Steroid-Refractory Acute GVHD to α1-Antitrypsin.Biol Blood Marrow Transplant. 2016 Sep;22(9):1596-1601. doi: 10.1016/j.bbmt.2016.05.011. Epub 2016 May 17. Biol Blood Marrow Transplant. 2016. PMID: 27223109 Clinical Trial.
-
A Phase 3, Single-Arm, Prospective Study of Remestemcel-L, Ex Vivo Culture-Expanded Adult Human Mesenchymal Stromal Cells for the Treatment of Pediatric Patients Who Failed to Respond to Steroid Treatment for Acute Graft-versus-Host Disease.Biol Blood Marrow Transplant. 2020 May;26(5):845-854. doi: 10.1016/j.bbmt.2020.01.018. Epub 2020 Feb 1. Biol Blood Marrow Transplant. 2020. PMID: 32018062 Free PMC article.
Cited by
-
Immune Suppression in Allogeneic Hematopoietic Stem Cell Transplantation.Handb Exp Pharmacol. 2022;272:209-243. doi: 10.1007/164_2021_544. Handb Exp Pharmacol. 2022. PMID: 34628553 Free PMC article.
-
Challenges and opportunities targeting mechanisms of epithelial injury and recovery in acute intestinal graft-versus-host disease.Mucosal Immunol. 2022 Apr;15(4):605-619. doi: 10.1038/s41385-022-00527-6. Epub 2022 Jun 2. Mucosal Immunol. 2022. PMID: 35654837 Free PMC article. Review.
-
A phase 1 trial of itacitinib, a selective JAK1 inhibitor, in patients with acute graft-versus-host disease.Blood Adv. 2020 Apr 28;4(8):1656-1669. doi: 10.1182/bloodadvances.2019001043. Blood Adv. 2020. PMID: 32324888 Free PMC article. Clinical Trial.
-
Alpha-1 Antitrypsin Deficiency and Pulmonary Morbidity in Patients with Primary Immunodeficiency Disease: A Single-Center Experience.Can Respir J. 2020 May 27;2020:4019608. doi: 10.1155/2020/4019608. eCollection 2020. Can Respir J. 2020. PMID: 32566054 Free PMC article.
-
A Review of Alpha-1 Antitrypsin Binding Partners for Immune Regulation and Potential Therapeutic Application.Int J Mol Sci. 2022 Feb 23;23(5):2441. doi: 10.3390/ijms23052441. Int J Mol Sci. 2022. PMID: 35269582 Free PMC article. Review.
References
-
- MacMillan ML, DeFor TE, Weisdorf DJ. The best endpoint for acute GVHD treatment trials. Blood. 2010;115(26):5412-5417. - PubMed
-
- MacMillan ML, Weisdorf DJ, Wagner JE, et al. . Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8(7):387-394. - PubMed
-
- MacMillan ML, Weisdorf DJ, Davies SM, et al. . Early antithymocyte globulin therapy improves survival in patients with steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8(1):40-46. - PubMed
-
- Khoury H, Kashyap A, Adkins DR, et al. . Treatment of steroid-resistant acute graft-versus-host disease with anti-thymocyte globulin. Bone Marrow Transplant. 2001;27(10):1059-1064. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

