T Cell Expression of C5a Receptor 2 Augments Murine Regulatory T Cell (TREG) Generation and TREG-Dependent Cardiac Allograft Survival
- PMID: 29436411
- PMCID: PMC5894525
- DOI: 10.4049/jimmunol.1701638
T Cell Expression of C5a Receptor 2 Augments Murine Regulatory T Cell (TREG) Generation and TREG-Dependent Cardiac Allograft Survival
Abstract
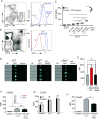
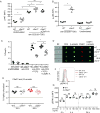
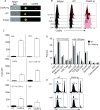
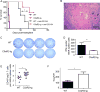
C5aR2 (C5L2/gp77) is a seven-transmembrane spanning receptor that binds to C5a but lacks motifs essential for G protein coupling and associated signal transduction. C5aR2 is expressed on immune cells, modulates various inflammatory diseases in mice, and has been shown to facilitate murine and human regulatory T cell (TREG) generation in vitro. Whether and how C5aR2 impacts in vivo TREG generation and pathogenic T cell-dependent disease models have not been established. In this article, we show that murine T cells express and upregulate C5aR2 during induced TREG (iTREG) generation and that the absence of T cell-expressed C5aR2 limits in vivo iTREG generation following adoptive transfer of naive CD4+ T cells into Rag1-/- recipients. Using newly generated C5aR2-transgenic mice, we show that overexpression of C5aR2 in naive CD4+ T cells augments in vivo iTREG generation. In a model of TREG-dependent cardiac allograft survival, recipient C5aR2 deficiency accelerates graft rejection associated with lower TREG/effector T cell ratios, whereas overexpression of C5aR2 in immune cells prolongs graft survival associated with an increase in TREG/effector T cell ratios. T cell-expressed C5aR2 modulates TREG induction without altering effector T cell proliferation or cytokine production. Distinct from reported findings in neutrophils and macrophages, TREG-expressed C5aR2 does not interact with β-arrestin or inhibit ERK1/2 signaling. Rather, cumulative evidence supports the conclusion that C5aR2 limits C5aR1-initiated signals known to inhibit TREG induction. Together, the data expand the role of C5aR2 in adaptive immunity by providing in vivo evidence that T cell-expressed C5aR2 physiologically modulates iTREG generation and iTREG-dependent allograft survival.
Copyright © 2018 by The American Association of Immunologists, Inc.
Conflict of interest statement
None
Figures




Similar articles
-
Monitoring C5aR2 Expression Using a Floxed tdTomato-C5aR2 Knock-In Mouse.J Immunol. 2017 Nov 1;199(9):3234-3248. doi: 10.4049/jimmunol.1700710. Epub 2017 Sep 1. J Immunol. 2017. PMID: 28864475
-
Cutting edge: Receptors for C3a and C5a modulate stability of alloantigen-reactive induced regulatory T cells.J Immunol. 2013 Jun 15;190(12):5921-5. doi: 10.4049/jimmunol.1300847. Epub 2013 May 20. J Immunol. 2013. PMID: 23690475 Free PMC article.
-
C5a receptors C5aR1 and C5aR2 mediate opposing pathologies in a mouse model of melanoma.FASEB J. 2019 Oct;33(10):11060-11071. doi: 10.1096/fj.201800980RR. Epub 2019 Jul 12. FASEB J. 2019. PMID: 31298935
-
The Complement Receptor C5aR2: A Powerful Modulator of Innate and Adaptive Immunity.J Immunol. 2019 Jun 15;202(12):3339-3348. doi: 10.4049/jimmunol.1900371. J Immunol. 2019. PMID: 31160390 Review.
-
The Controversial C5a Receptor C5aR2: Its Role in Health and Disease.J Immunol Res. 2017;2017:8193932. doi: 10.1155/2017/8193932. Epub 2017 Jun 15. J Immunol Res. 2017. PMID: 28706957 Free PMC article. Review.
Cited by
-
The "C3aR Antagonist" SB290157 is a Partial C5aR2 Agonist.Front Pharmacol. 2021 Jan 21;11:591398. doi: 10.3389/fphar.2020.591398. eCollection 2020. Front Pharmacol. 2021. PMID: 33551801 Free PMC article.
-
An integrated analysis of C5AR2 related to malignant properties and immune infiltration of gliomas.Cancer Innov. 2022 Oct 5;1(3):240-251. doi: 10.1002/cai2.29. eCollection 2022 Oct. Cancer Innov. 2022. PMID: 38089762 Free PMC article.
-
Role of Complement Activation in Allograft Inflammation.Curr Transplant Rep. 2019 Mar;6(1):52-59. doi: 10.1007/s40472-019-0224-2. Epub 2019 Jan 15. Curr Transplant Rep. 2019. PMID: 31673484 Free PMC article.
-
The Role of Complement in Organ Transplantation.Front Immunol. 2019 Oct 4;10:2380. doi: 10.3389/fimmu.2019.02380. eCollection 2019. Front Immunol. 2019. PMID: 31636644 Free PMC article. Review.
-
When rubber meets the road: how innate features of adaptive immune cells play critical roles in transplant alloimmunity.Curr Opin Organ Transplant. 2019 Dec;24(6):659-663. doi: 10.1097/MOT.0000000000000706. Curr Opin Organ Transplant. 2019. PMID: 31577597 Free PMC article. Review.
References
-
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. - PubMed
-
- Kronenberg M, Rudensky A. Regulation of immunity by self-reactive T cells. Nature. 2005;435:598–604. - PubMed
-
- Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

