Improvement of Antitumor Therapies Based on Vaccines and Immune-Checkpoint Inhibitors by Counteracting Tumor-Immunostimulation
- PMID: 29435437
- PMCID: PMC5790794
- DOI: 10.3389/fonc.2018.00006
Improvement of Antitumor Therapies Based on Vaccines and Immune-Checkpoint Inhibitors by Counteracting Tumor-Immunostimulation
Abstract
Immune-checkpoint inhibitors and antitumor vaccines may produce both tumor-inhibitory and tumor-stimulatory effects on growing tumors depending on the stage of tumor growth at which treatment is initiated. These paradoxical results are not necessarily incompatible with current tumor immunology but they might better be explained assuming the involvement of the phenomenon of tumor immunostimulation. This phenomenon was originally postulated on the basis that the immune response (IR) evoked in Winn tests by strong chemical murine tumors was not linear but biphasic, with strong IR producing inhibition and weak IR inducing stimulation of tumor growth. Herein, we extended those former observations to weak spontaneous murine tumors growing in pre-immunized, immune-competent and immune-depressed mice. Furthermore, we demonstrated that the interaction of specifical T cells and target tumor cells at low stimulatory ratios enhanced the production of chemokines aimed to recruit macrophages at the tumor site, which, upon activation of toll-like receptor 4 and p38 signaling pathways, would recruit and activate more macrophages and other inflammatory cells which would produce growth-stimulating signals leading to an accelerated tumor growth. On this basis, the paradoxical effects achieved by immunological therapies on growing tumors could be explained depending upon where the therapy-induced IR stands on the biphasic IR curve at each stage of tumor growth. At stages where tumor growth was enhanced (medium and large-sized tumors), counteraction of the tumor-immunostimulatory effect with anti-inflammatory strategies or, more efficiently, with selective inhibitors of p38 signaling pathways enabled the otherwise tumor-promoting immunological strategies to produce significant inhibition of tumor growth.
Keywords: antitumor vaccines; immune-checkpoints inhibitors; immunosurveillance; murine tumors; tumor-immunostimulation.
Figures
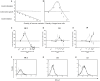
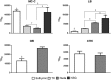
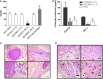

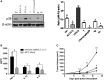
Similar articles
-
Inhibition of hyperprogressive cancer disease induced by immune-checkpoint blockade upon co-treatment with meta-tyrosine and p38 pathway inhibitor.BMC Cancer. 2022 Aug 3;22(1):845. doi: 10.1186/s12885-022-09941-2. BMC Cancer. 2022. PMID: 35922755 Free PMC article.
-
G3139 and other CpG-containing immunostimulatory phosphorothioate oligodeoxynucleotides are potent suppressors of the growth of human tumor xenografts in nude mice.Oligonucleotides. 2006 Spring;16(1):83-93. doi: 10.1089/oli.2006.16.83. Oligonucleotides. 2006. PMID: 16584297
-
On the immunostimulatory hypothesis of cancer.Medicina (B Aires). 2011;71(6):509-13. Medicina (B Aires). 2011. PMID: 22167722
-
Clinical impact of checkpoint inhibitors as novel cancer therapies.Drugs. 2014 Nov;74(17):1993-2013. doi: 10.1007/s40265-014-0305-6. Drugs. 2014. PMID: 25344022 Free PMC article. Review.
-
Emerging strategies for combination checkpoint modulators in cancer immunotherapy.J Clin Invest. 2018 Aug 1;128(8):3209-3218. doi: 10.1172/JCI120775. Epub 2018 Aug 1. J Clin Invest. 2018. PMID: 30067248 Free PMC article. Review.
Cited by
-
Inhibition of hyperprogressive cancer disease induced by immune-checkpoint blockade upon co-treatment with meta-tyrosine and p38 pathway inhibitor.BMC Cancer. 2022 Aug 3;22(1):845. doi: 10.1186/s12885-022-09941-2. BMC Cancer. 2022. PMID: 35922755 Free PMC article.
-
Advances in the Study of Hyperprogression of Different Tumors Treated with PD-1/PD-L1 Antibody and the Mechanisms of Its Occurrence.Cancers (Basel). 2023 Feb 18;15(4):1314. doi: 10.3390/cancers15041314. Cancers (Basel). 2023. PMID: 36831655 Free PMC article. Review.
-
Inflammation Control and Immunotherapeutic Strategies in Comprehensive Cancer Treatment.Metabolites. 2023 Jan 13;13(1):123. doi: 10.3390/metabo13010123. Metabolites. 2023. PMID: 36677048 Free PMC article.
-
Impact of endogenous glucocorticoid on response to immune checkpoint blockade in patients with advanced cancer.Front Immunol. 2023 Apr 11;14:1081790. doi: 10.3389/fimmu.2023.1081790. eCollection 2023. Front Immunol. 2023. PMID: 37114049 Free PMC article.
References
-
- Prehn RT, Main JM. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst (1957) 18:769.tyjl–78.tyjl. - PubMed
-
- Janeway CA, Travers P, Walport M, Shlomchik MJ. Manipulation of the immune response. In: Janeway CA, editor. Immunobiology. New York: Garland; (2001). p. 566–77.
LinkOut - more resources
Full Text Sources
Other Literature Sources

