Identification of a novel autoantibody against self-vimentin specific in secondary Sjögren's syndrome
- PMID: 29433534
- PMCID: PMC5810024
- DOI: 10.1186/s13075-017-1508-5
Identification of a novel autoantibody against self-vimentin specific in secondary Sjögren's syndrome
Abstract
Background: Sjögren's syndrome (SS) is a primary autoimmune disease (pSS) or secondarily associated with other autoimmune diseases (sSS). The mechanisms underlying immune dysregulation in this syndrome remain unknown, and clinically it is difficult to diagnose owing to a lack of specific biomarkers.
Methods: We extracted immunoglobulins (Igs) from the sera of patients with sSS associated with rheumatoid arthritis (RA) and used them to screen a phage display library of peptides with random sequences.
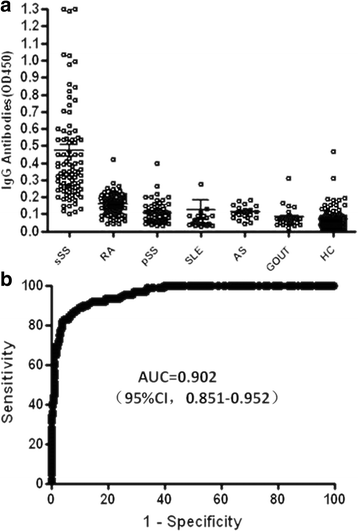
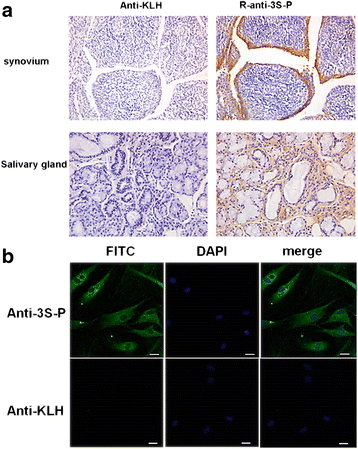
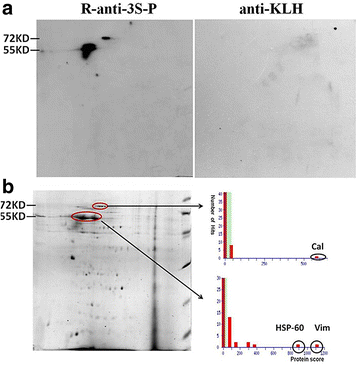
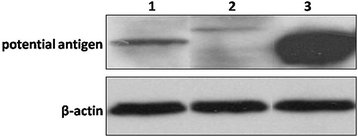
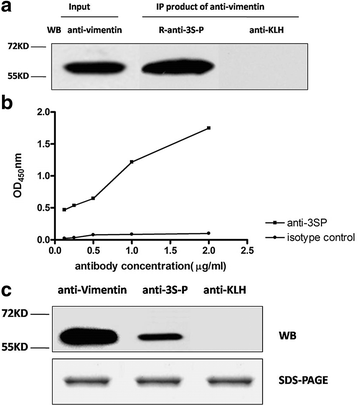
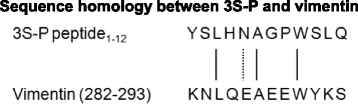
Results: Our results show that an sSS-specific peptide, designated 3S-P, was recognized by sera of 68.2% (60 of 88) patients with sSS, 66.2% of patients with RA-sSS, and 76.5% of patients with systemic lupus erythematosus (SLE)-sSS. The anti-3S-P antibody was scarcely found in patients with pSS (1.8%), RA (1.3%), SLE (4.2%), ankylosing spondylitis (0%), and gout (3.3%), as well as in healthy donors (2%). The 3S-P-binding Igs (antibodies) were used to identify antigens from salivary glands and synovial tissues from patients with sSS. A putative target autoantigen expressed in the synovium and salivary gland recognized by anti-3S-P antibody was identified as self-vimentin.
Conclusions: This novel autoantibody is highly specific in the diagnosis of sSS, and the underlying molecular mechanism of the disease might be epitope spreading involved with vimentin.
Keywords: Autoantigen; Biomarker; Sjögren’s syndrome; Vimentin.
Conflict of interest statement
Ethics approval and consent to participate
All the procedures involving specimens obtained from human subjects were performed under protocols approved by the Human Research Protective Committee of People’s Hospital Peking University, and all the methods were carried out in accordance with the guidelines and regulations of the Ethics Committee of People’s Hospital Peking University. Informed consent was also provided by all subjects before the study protocol was initiated.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Sjögren's syndrome and systemic lupus erythematosus: links and risks.Open Access Rheumatol. 2019 Jan 29;11:33-45. doi: 10.2147/OARRR.S167783. eCollection 2019. Open Access Rheumatol. 2019. PMID: 30774485 Free PMC article. Review.
-
Comparative analysis of novel autoantibody isotypes against citrullinated-inter-alpha-trypsin inhibitor heavy chain 3 (ITIH3)(542-556) peptide in serum from Taiwanese females with rheumatoid arthritis, primary Sjögren's syndrome and secondary Sjögren's syndrome in rheumatoid arthritis.J Proteomics. 2016 Jun 1;141:1-11. doi: 10.1016/j.jprot.2016.03.031. Epub 2016 Apr 9. J Proteomics. 2016. PMID: 27072115
-
The epitope study of alpha-fodrin autoantibody in primary Sjögren's syndrome.Clin Exp Immunol. 2007 Sep;149(3):497-503. doi: 10.1111/j.1365-2249.2007.03435.x. Epub 2007 Jul 5. Clin Exp Immunol. 2007. PMID: 17614976 Free PMC article.
-
The role of muscarinic acetylcholine receptor type 3 polypeptide (M3RP205-220) antibody in the saliva of patients with primary Sjögren's syndrome.Clin Exp Rheumatol. 2012 May-Jun;30(3):322-6. Epub 2012 Jun 25. Clin Exp Rheumatol. 2012. PMID: 22510450
-
Secreted autoantibody repertoires in Sjögren's syndrome and systemic lupus erythematosus: A proteomic approach.Autoimmun Rev. 2016 Apr;15(4):405-10. doi: 10.1016/j.autrev.2016.01.008. Epub 2016 Jan 22. Autoimmun Rev. 2016. PMID: 26804757 Review.
Cited by
-
Oral Lichen Planus and Lichenoid Lesions in Sjogren's Syndrome Patients: A Prospective Study.Int J Dent. 2019 May 7;2019:1603657. doi: 10.1155/2019/1603657. eCollection 2019. Int J Dent. 2019. PMID: 31205471 Free PMC article.
-
Anti-vimentin antibodies are associated with higher severity of Sjögren's disease.Clin Immunol. 2023 Feb;247:109243. doi: 10.1016/j.clim.2023.109243. Epub 2023 Jan 24. Clin Immunol. 2023. PMID: 36702181 Free PMC article.
-
Sjögren's syndrome and systemic lupus erythematosus: links and risks.Open Access Rheumatol. 2019 Jan 29;11:33-45. doi: 10.2147/OARRR.S167783. eCollection 2019. Open Access Rheumatol. 2019. PMID: 30774485 Free PMC article. Review.
-
Tissue-Specific Autoantibodies Improve Diagnosis of Primary Sjögren's Syndrome in the Early Stage and Indicate Localized Salivary Injury.J Immunol Res. 2019 May 7;2019:3642937. doi: 10.1155/2019/3642937. eCollection 2019. J Immunol Res. 2019. PMID: 31205955 Free PMC article.
-
Poria Acid, Triterpenoids Extracted from Poria cocos, Inhibits the Invasion and Metastasis of Gastric Cancer Cells.Molecules. 2022 Jun 6;27(11):3629. doi: 10.3390/molecules27113629. Molecules. 2022. PMID: 35684565 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

