Dissecting the biochemical architecture and morphological release pathways of the human platelet extracellular vesiculome
- PMID: 29427073
- PMCID: PMC11105464
- DOI: 10.1007/s00018-018-2771-6
Dissecting the biochemical architecture and morphological release pathways of the human platelet extracellular vesiculome
Abstract
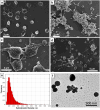
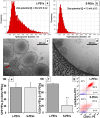
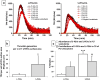
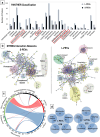
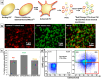
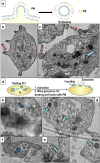
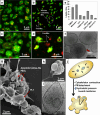
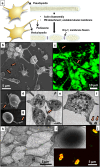
Platelet extracellular vesicles (PEVs) have emerged as potential mediators in intercellular communication. PEVs exhibit several activities with pathophysiological importance and may serve as diagnostic biomarkers. Here, imaging and analytical techniques were employed to unveil morphological pathways of the release, structure, composition, and surface properties of PEVs derived from human platelets (PLTs) activated with the thrombin receptor activating peptide (TRAP). Based on extensive electron microscopy analysis, we propose four morphological pathways for PEVs release from TRAP-activated PLTs: (1) plasma membrane budding, (2) extrusion of multivesicular α-granules and cytoplasmic vacuoles, (3) plasma membrane blistering and (4) "pearling" of PLT pseudopodia. The PLT extracellular vesiculome encompasses ectosomes, exosomes, free mitochondria, mitochondria-containing vesicles, "podiasomes" and PLT "ghosts". Interestingly, a flow cytometry showed a population of TOM20+LC3+ PEVs, likely products of platelet mitophagy. We found that lipidomic and proteomic profiles were different between the small PEV (S-PEVs; mean diameter 103 nm) and the large vesicle (L-PEVs; mean diameter 350 nm) fractions separated by differential centrifugation. In addition, the majority of PEVs released by activated PLTs was composed of S-PEVs which have markedly higher thrombin generation activity per unit of PEV surface area compared to L-PEVs, and contribute approximately 60% of the PLT vesiculome procoagulant potency.
Keywords: Ectosome; Exosome; Extracellular vesicles; Membrane microparticles; Membrane pearling; Mitophagy; Platelet; Thrombin generation.
Figures
Similar articles
-
Structural analysis of platelet fragments and extracellular vesicles produced by apheresis platelets during storage.Blood Adv. 2024 Jan 9;8(1):207-218. doi: 10.1182/bloodadvances.2023011325. Blood Adv. 2024. PMID: 37967384 Free PMC article.
-
Platelet-released extracellular vesicles: the effects of thrombin activation.Cell Mol Life Sci. 2022 Mar 14;79(3):190. doi: 10.1007/s00018-022-04222-4. Cell Mol Life Sci. 2022. PMID: 35288766 Free PMC article.
-
Platelet extracellular vesicles are efficient delivery vehicles of doxorubicin, an anti-cancer drug: preparation and in vitro characterization.Platelets. 2023 Dec;34(1):2237134. doi: 10.1080/09537104.2023.2237134. Platelets. 2023. PMID: 37580876
-
Platelets Extracellular Vesicles as Regulators of Cancer Progression-An Updated Perspective.Int J Mol Sci. 2020 Jul 22;21(15):5195. doi: 10.3390/ijms21155195. Int J Mol Sci. 2020. PMID: 32707975 Free PMC article. Review.
-
Extracellular vesicles from activated platelets: a semiquantitative cryo-electron microscopy and immuno-gold labeling study.Platelets. 2017 May;28(3):263-271. doi: 10.1080/09537104.2016.1268255. Epub 2017 Jan 19. Platelets. 2017. PMID: 28102751 Review.
Cited by
-
Structural analysis of platelet fragments and extracellular vesicles produced by apheresis platelets during storage.Blood Adv. 2024 Jan 9;8(1):207-218. doi: 10.1182/bloodadvances.2023011325. Blood Adv. 2024. PMID: 37967384 Free PMC article.
-
Activation mechanism dependent surface exposure of cellular factor XIII on activated platelets and platelet microparticles.J Thromb Haemost. 2022 May;20(5):1223-1235. doi: 10.1111/jth.15668. Epub 2022 Feb 21. J Thromb Haemost. 2022. PMID: 35146910 Free PMC article.
-
Mode of induction of platelet-derived extracellular vesicles is a critical determinant of their phenotype and function.Sci Rep. 2020 Oct 22;10(1):18061. doi: 10.1038/s41598-020-73005-3. Sci Rep. 2020. PMID: 33093473 Free PMC article.
-
Massive Release of CD9+ Microvesicles in Human Immunodeficiency Virus Infection, Regardless of Virologic Control.J Infect Dis. 2022 Mar 15;225(6):1040-1049. doi: 10.1093/infdis/jiaa375. J Infect Dis. 2022. PMID: 32603406 Free PMC article.
-
Platelet Microparticles Protect Acute Myelogenous Leukemia Cells against Daunorubicin-Induced Apoptosis.Cancers (Basel). 2021 Apr 14;13(8):1870. doi: 10.3390/cancers13081870. Cancers (Basel). 2021. PMID: 33919720 Free PMC article.
References
-
- Tkach M, Thery C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164:1226–1232. - PubMed
-
- Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25:364–372. - PubMed
-
- Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–288. - PubMed
-
- Phipps RP. Platelets at the interface between thrombosis, inflammation and immunity. Thromb Res. 2011;127:179. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

