Differences in Local and Systemic TFV PK Among Premenopausal Versus Postmenopausal Women Exposed to TFV 1% Vaginal Gel
- PMID: 29424790
- PMCID: PMC5902131
- DOI: 10.1097/QAI.0000000000001648
Differences in Local and Systemic TFV PK Among Premenopausal Versus Postmenopausal Women Exposed to TFV 1% Vaginal Gel
Abstract
Objective: We describe and compare the local and systemic pharmacokinetics (PK) of tenofovir (TFV) and TFV-diphosphate (TFV-DP) in healthy premenopausal (PRE) and postmenopausal (POST) women using TFV 1% gel and correlate local PK with other mucosal end points.
Methods: PRE (n = 20) and POST (n = 17) women used 2 doses of TFV 1% vaginal gel, separated by 2 hours. Blood and cervicovaginal samples were obtained 3 and 23 hours after the second dose. PRE women used gel in the follicular and luteal phases of the menstrual cycle. POST women used gel at baseline and again after approximately 2 months of treatment with 0.01% vaginal estradiol (E2) cream.
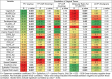
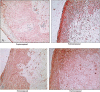
Results: Median TFV concentrations in cervicovaginal aspirate (ng/mL) and vaginal tissue (ng/mg) were significantly higher in PRE (4.3E10, 49.8) versus POST women (2.6E10, 2.2). POST women had significantly higher median molecular ratios of TFV-DP to TFV (3.7%) compared with PRE (0.19%). After vaginal E2 treatment, the local and systemic PK end points in POST women were generally similar to PRE women (all P values > 0.05). Importantly, median vaginal tissue TFV-DP concentrations (fmol/mg) among PRE, POST, and POST women after E2 therapy were similar (292.5, 463.3, and 184.6, respectively). Vaginal tissue TFV concentrations were significantly positively correlated with vaginal epithelial thickness, whereas vaginal tissue TFV-DP concentrations were positively correlated with density of vaginal CD4 and CD8 immune cells.
Conclusions: The state of the cervicovaginal mucosa has a significant impact on local and systemic PK of a topically applied microbicide.
Conflict of interest statement
The authors have no funding or conflicts of interest to disclose.
Figures
Similar articles
-
Vaginal microbiota and mucosal pharmacokinetics of tenofovir in healthy women using tenofovir and tenofovir/levonorgestrel vaginal rings.PLoS One. 2019 May 20;14(5):e0217229. doi: 10.1371/journal.pone.0217229. eCollection 2019. PLoS One. 2019. PMID: 31107913 Free PMC article. Clinical Trial.
-
Pharmacokinetics and Pharmacodynamics of Tenofovir Reduced-Glycerin 1% Gel in the Rectal and Vaginal Compartments in Women: A Cross-Compartmental Study With Directly Observed Dosing.J Acquir Immune Defic Syndr. 2018 Jun 1;78(2):175-182. doi: 10.1097/QAI.0000000000001655. J Acquir Immune Defic Syndr. 2018. PMID: 29767639 Free PMC article. Clinical Trial.
-
Effect of Hormonal Contraception on Pharmacokinetics of Vaginal Tenofovir in Healthy Women: Increased Tenofovir Diphosphate in Injectable Depot Medroxyprogesterone Acetate Users.J Acquir Immune Defic Syndr. 2019 Jan 1;80(1):79-88. doi: 10.1097/QAI.0000000000001864. J Acquir Immune Defic Syndr. 2019. PMID: 30212395 Clinical Trial.
-
The Vaginal Microbiome and its Potential to Impact Efficacy of HIV Pre-exposure Prophylaxis for Women.Curr HIV/AIDS Rep. 2017 Oct;14(5):153-160. doi: 10.1007/s11904-017-0362-z. Curr HIV/AIDS Rep. 2017. PMID: 28812207 Free PMC article. Review.
-
Recent advances on anti-HIV vaginal delivery systems development.Adv Drug Deliv Rev. 2015 Sep 15;92:123-45. doi: 10.1016/j.addr.2015.03.015. Epub 2015 Apr 7. Adv Drug Deliv Rev. 2015. PMID: 25858666 Review.
Cited by
-
Irreversible depletion of intestinal CD4+ T cells is associated with T cell activation during chronic HIV infection.JCI Insight. 2021 Nov 22;6(22):e146162. doi: 10.1172/jci.insight.146162. JCI Insight. 2021. PMID: 34618690 Free PMC article.
-
First-in-human study to assess the pharmacokinetics, tolerability, and safety of single-dose oxybutynin hydrochloride administered via a microprocessor-controlled intravaginal ring.Drug Deliv. 2023 Dec;30(1):2180113. doi: 10.1080/10717544.2023.2180113. Drug Deliv. 2023. PMID: 36815245 Free PMC article.
-
Vaginal microbiota and mucosal pharmacokinetics of tenofovir in healthy women using tenofovir and tenofovir/levonorgestrel vaginal rings.PLoS One. 2019 May 20;14(5):e0217229. doi: 10.1371/journal.pone.0217229. eCollection 2019. PLoS One. 2019. PMID: 31107913 Free PMC article. Clinical Trial.
-
A phase 1/2, open-label, parallel group study to evaluate the safety and pharmacokinetics of DARE-HRT1 (80 μg estradiol/4 mg progesterone and 160 μg estradiol/8 mg progesterone intravaginal rings) over 12 weeks in healthy postmenopausal women.Menopause. 2023 Aug 1;30(8):817-823. doi: 10.1097/GME.0000000000002210. Epub 2023 Jun 20. Menopause. 2023. PMID: 37339390 Free PMC article. Clinical Trial.
-
Antiretroviral therapy and vaginally administered contraceptive hormones: a three-arm, pharmacokinetic study.Lancet HIV. 2019 Sep;6(9):e601-e612. doi: 10.1016/S2352-3018(19)30155-9. Lancet HIV. 2019. PMID: 31498109 Free PMC article.
References
-
- Abdool Karim SS, Baxter C. Microbicides for prevention of HIV infection: clinical efficacy trials. Curr Top Microbiol Immunol. 2014;383:97–115. - PubMed
-
- United Nations. Prevention Gap Report. 2016. Available at: http://www.unaids.org/sites/default/files/media_asset/2016-prevention-ga....
-
- Reichelderfer PS, Coombs RW, Wright DJ, et al. Effect of menstrual cycle on HIV-1 levels in the peripheral blood and genital tract. WHS 001 Study Team. AIDS. 2000;14:2101–2107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

