Identification of a Potential Common Ancestor for Mammalian Cross-Presenting Dendritic Cells in Teleost Respiratory Surfaces
- PMID: 29422901
- PMCID: PMC5788898
- DOI: 10.3389/fimmu.2018.00059
Identification of a Potential Common Ancestor for Mammalian Cross-Presenting Dendritic Cells in Teleost Respiratory Surfaces
Abstract
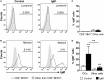
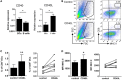
Dendritic cells (DCs) are highly specialized antigen-presenting cells that bridge innate and adaptive immune responses in vertebrates, being key modulators in the initiation of specific responses. Although teleost fish present the main elements of a fully developed adaptive immune system, not many studies have focused on identifying specific DC subsets in teleost species. Previous work from our group identified in rainbow trout (Oncorhynchus mykiss) skin a DC subpopulation co-expressing CD8α and major histocompatibility complex II β on the cell surface. Interestingly, these CD8+ DCs expressed common unique markers of mammalian cross-presenting DCs, a DC subset with an important role in antigen presentation and activation of CD8+ T cytotoxic lymphocytes. In this study, we have identified a similar DC subset in rainbow trout gills that also transcribes molecules uniquely expressed on diverse mammalian cross-presenting DC populations such as CD8, CD103, CD141, Batf3, IFN regulatory protein 8, and toll-like receptor 3. Hence, we have undertaken a broad phenotypic and functional characterization of this new DC subset that includes the confirmation of novel capacities for DCs in teleost, such an IgM-binding capacity and responsiveness to CD40 ligand. Furthermore, our results show that in gills, this DC subset shows some different phenotypic and functional characteristics when compared with their homologs in the skin, suggesting an adaptation of the cells to different mucosal tissues or different maturation status depending on their location. Our findings contribute to increase our knowledge on fish cross-presenting DCs, an important cell population to take into account for the future design of mucosal vaccination strategies.
Keywords: CD8; cross-presentation; dendritic cells; gills; major histocompatibility complex II; rainbow trout; respiratory surfaces.
Figures
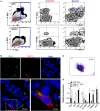

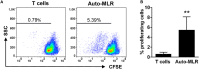
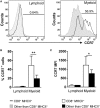
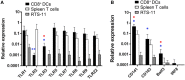
Similar articles
-
Identification of CD8α+ dendritic cells in rainbow trout (Oncorhynchus mykiss) intestine.Fish Shellfish Immunol. 2019 Jun;89:309-318. doi: 10.1016/j.fsi.2019.04.001. Epub 2019 Apr 6. Fish Shellfish Immunol. 2019. PMID: 30959183 Free PMC article.
-
Identification of Teleost Skin CD8α+ Dendritic-like Cells, Representing a Potential Common Ancestor for Mammalian Cross-Presenting Dendritic Cells.J Immunol. 2015 Aug 15;195(4):1825-37. doi: 10.4049/jimmunol.1500322. Epub 2015 Jul 15. J Immunol. 2015. PMID: 26179908
-
Dendritic type, accessory cells within the mammalian thymic microenvironment. Antigen presentation in the dendritic neuro-endocrine-immune cellular network.In Vivo. 1997 Jul-Aug;11(4):351-70. In Vivo. 1997. PMID: 9292303
-
Unique functions of splenic CD8alpha+ dendritic cells during infection with intracellular pathogens.Immunol Lett. 2007 Dec 15;114(2):66-72. doi: 10.1016/j.imlet.2007.09.007. Epub 2007 Oct 12. Immunol Lett. 2007. PMID: 17964665 Review.
-
Evolutionary and functional relationships of B cells from fish and mammals: insights into their novel roles in phagocytosis and presentation of particulate antigen.Infect Disord Drug Targets. 2012 Jun;12(3):200-12. doi: 10.2174/187152612800564419. Infect Disord Drug Targets. 2012. PMID: 22394174 Free PMC article. Review.
Cited by
-
NF-κB signaling induces inductive expression of the downstream molecules and IgD gene in the freshwater carp, Catla catla.3 Biotech. 2020 Oct;10(10):445. doi: 10.1007/s13205-020-02435-7. Epub 2020 Sep 19. 3 Biotech. 2020. PMID: 33014688 Free PMC article.
-
Cluster of differentiation antigens: essential roles in the identification of teleost fish T lymphocytes.Mar Life Sci Technol. 2022 Aug 19;4(3):303-316. doi: 10.1007/s42995-022-00136-z. eCollection 2022 Aug. Mar Life Sci Technol. 2022. PMID: 37073166 Free PMC article. Review.
-
Antigen Presentation and Autophagy in Teleost Adaptive Immunity.Int J Mol Sci. 2022 Apr 28;23(9):4899. doi: 10.3390/ijms23094899. Int J Mol Sci. 2022. PMID: 35563287 Free PMC article. Review.
-
Identification of CD8α+ dendritic cells in rainbow trout (Oncorhynchus mykiss) intestine.Fish Shellfish Immunol. 2019 Jun;89:309-318. doi: 10.1016/j.fsi.2019.04.001. Epub 2019 Apr 6. Fish Shellfish Immunol. 2019. PMID: 30959183 Free PMC article.
-
Mechanisms of Fish Macrophage Antimicrobial Immunity.Front Immunol. 2018 May 28;9:1105. doi: 10.3389/fimmu.2018.01105. eCollection 2018. Front Immunol. 2018. PMID: 29892285 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

