PML: Regulation and multifaceted function beyond tumor suppression
- PMID: 29416846
- PMCID: PMC5785837
- DOI: 10.1186/s13578-018-0204-8
PML: Regulation and multifaceted function beyond tumor suppression
Erratum in
-
Correction to: PML: Regulation and multifaceted function beyond tumor suppression.Cell Biosci. 2018 Mar 6;8:18. doi: 10.1186/s13578-018-0213-7. eCollection 2018. Cell Biosci. 2018. PMID: 29528047 Free PMC article.
Abstract
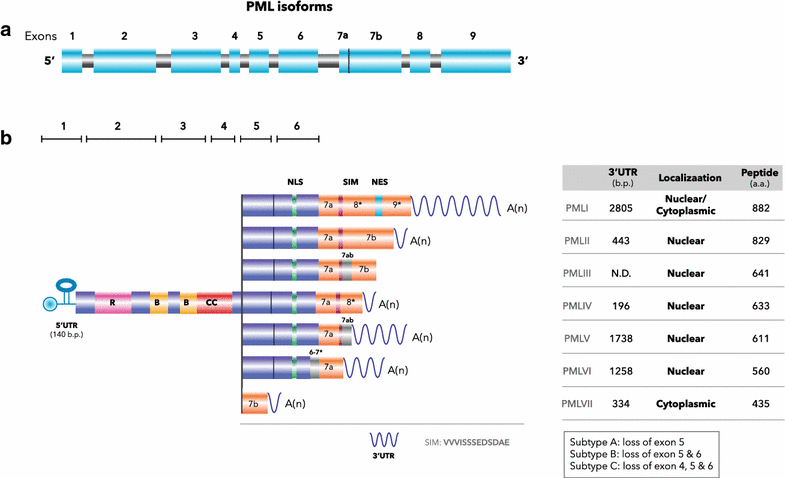
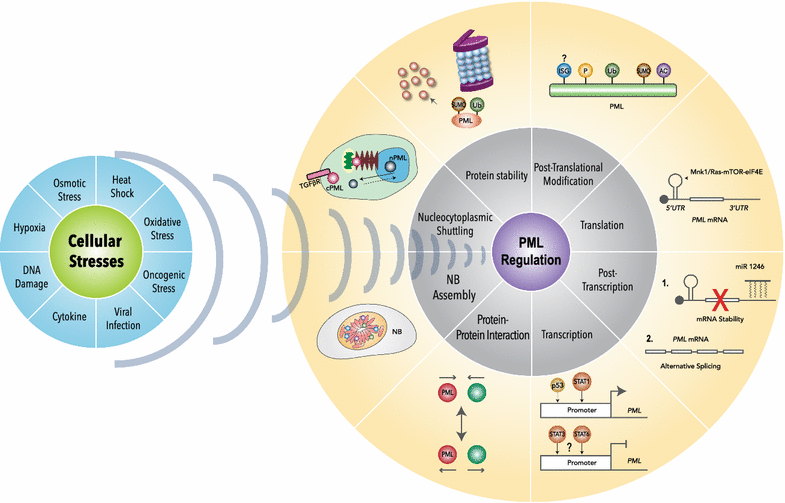
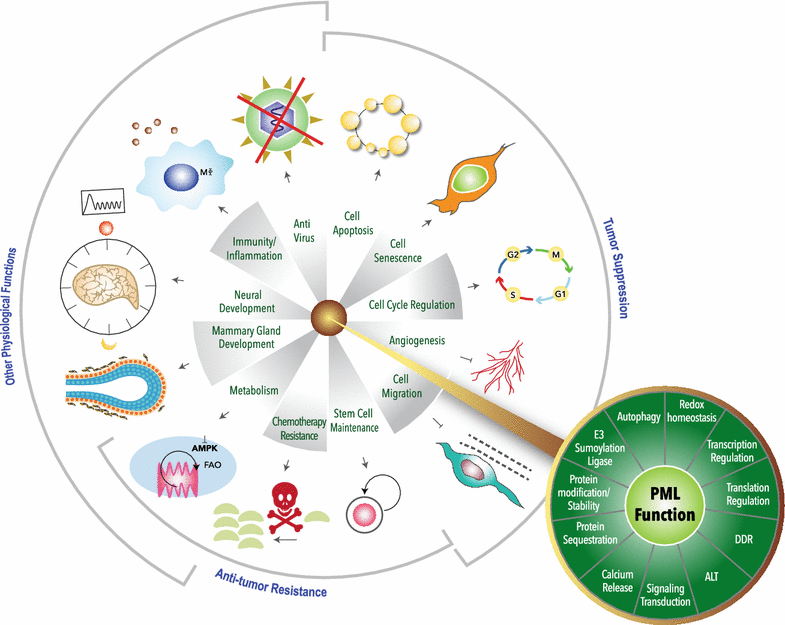
Promyelocytic leukemia protein (PML) was originally identified as a fusion partner of retinoic acid receptor alpha in acute promyelocytic leukemia patients with the (15;17) chromosomal translocation, giving rise to PML-RARα and RARα-PML fusion proteins. A body of evidence indicated that PML possesses tumor suppressing activity by regulating apoptosis, cell cycle, senescence and DNA damage responses. PML is enriched in discrete nuclear substructures in mammalian cells with 0.2-1 μm diameter in size, referred to as alternately Kremer bodies, nuclear domain 10, PML oncogenic domains or PML nuclear bodies (NBs). Dysregulation of PML NB formation results in altered transcriptional regulation, protein modification, apoptosis and cellular senescence. In addition to PML NBs, PML is also present in nucleoplasm and cytoplasmic compartments, including the endoplasmic reticulum and mitochondria-associated membranes. The role of PML in tumor suppression has been extensively studied but increasing evidence indicates that PML also plays versatile roles in stem cell renewal, metabolism, inflammatory responses, neural function, mammary development and angiogenesis. In this review, we will briefly describe the known PML regulation and function and include new findings.
Keywords: Angiogenesis; Chemotherapy resistance; Gene expression; Inflammatory responses; Mammary development; Metabolism; Neural function; PML; Protein modification; Proteolysis; Stem cell and cancer stem cell renewal.
Figures
Similar articles
-
The functional roles of PML nuclear bodies in genome maintenance.Mutat Res. 2018 May;809:99-107. doi: 10.1016/j.mrfmmm.2017.05.002. Epub 2017 May 5. Mutat Res. 2018. PMID: 28521962 Review.
-
The function, regulation and therapeutic implications of the tumor suppressor protein, PML.Cell Biosci. 2015 Nov 4;5:60. doi: 10.1186/s13578-015-0051-9. eCollection 2015. Cell Biosci. 2015. PMID: 26539288 Free PMC article. Review.
-
Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment, and As2O3-induced PML or PML/retinoic acid receptor alpha degradation.J Exp Med. 2001 Jun 18;193(12):1361-71. doi: 10.1084/jem.193.12.1361. J Exp Med. 2001. PMID: 11413191 Free PMC article.
-
Role of nuclear bodies in apoptosis signalling.Biochim Biophys Acta. 2008 Nov;1783(11):2185-94. doi: 10.1016/j.bbamcr.2008.07.002. Epub 2008 Jul 16. Biochim Biophys Acta. 2008. PMID: 18680765 Review.
-
Involvement of PML-I in reformation of PML nuclear bodies in acute promyelocytic leukemia cells by leptomycin B.Toxicol Appl Pharmacol. 2019 Dec 1;384:114775. doi: 10.1016/j.taap.2019.114775. Epub 2019 Nov 6. Toxicol Appl Pharmacol. 2019. PMID: 31669778
Cited by
-
JAK2V617F myeloproliferative neoplasm eradication by a novel interferon/arsenic therapy involves PML.J Exp Med. 2021 Feb 1;218(2):e20201268. doi: 10.1084/jem.20201268. J Exp Med. 2021. PMID: 33075130 Free PMC article.
-
MALL, a membrane-tetra-spanning proteolipid overexpressed in cancer, is present in membraneless nuclear biomolecular condensates.Cell Mol Life Sci. 2022 Apr 10;79(5):236. doi: 10.1007/s00018-022-04270-w. Cell Mol Life Sci. 2022. PMID: 35399121 Free PMC article.
-
Liquid‒liquid phase separation: roles and implications in future cancer treatment.Int J Biol Sci. 2023 Aug 6;19(13):4139-4156. doi: 10.7150/ijbs.81521. eCollection 2023. Int J Biol Sci. 2023. PMID: 37705755 Free PMC article. Review.
-
Molecular Cloning of Alternative Splicing Variants of the Porcine PML Gene and Its Expression Patterns During Japanese Encephalitis Virus Infection.Front Vet Sci. 2021 Nov 23;8:757978. doi: 10.3389/fvets.2021.757978. eCollection 2021. Front Vet Sci. 2021. PMID: 34888375 Free PMC article.
-
Correction to: PML: Regulation and multifaceted function beyond tumor suppression.Cell Biosci. 2018 Mar 6;8:18. doi: 10.1186/s13578-018-0213-7. eCollection 2018. Cell Biosci. 2018. PMID: 29528047 Free PMC article.
References
-
- Kakizuka A, Miller WH, Jr, Umesono K, Warrell RP, Jr, Frankel SR, Murty VVVS, Dmitrovsky E, Evans RM. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RARα with a novel putative transcription factor. PML Cell. 1991;66:663–674. doi: 10.1016/0092-8674(91)90112-C. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

