Clinical efficacy and safety of afatinib in the treatment of non-small-cell lung cancer in Chinese patients
- PMID: 29416353
- PMCID: PMC5790073
- DOI: 10.2147/OTT.S136579
Clinical efficacy and safety of afatinib in the treatment of non-small-cell lung cancer in Chinese patients
Abstract
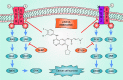
Compared with various malignant tumors, lung cancer has high incidence and the highest mortality worldwide. Non-small-cell lung cancer (NSCLC), the most common kind of lung cancer, is still a great threat to the world, including China. Surgery, platinum-based chemotherapy, and radiotherapy are still the primary treatments for NSCLC patients in the clinic, whereas immunotherapy and targeted therapy are gradually playing more important roles. A next-generation tyrosine kinase inhibitor (TKI), afatinib, was developed as a targeted reagent for epidermal growth factor receptor (EGFR). This targeted drug was effective in a series of trials. The US Food and Drug Administration then approved afatinib as a new first-line treatment for EGFR L858R and exon 19 deletion mutant patients in 2013. This review focused on current clinical studies of afatinib. Although this TKI was not widely available in China until recently, we aim to provide a reference for its future use in China.
Keywords: Chinese patients; NSCLC; afatinib; companion diagnosis; drug resistance; side effect.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Afatinib for the treatment of metastatic non-small cell lung cancer.Cancer Manag Res. 2015 Feb 19;7:75-82. doi: 10.2147/CMAR.S51808. eCollection 2015. Cancer Manag Res. 2015. PMID: 25733926 Free PMC article. Review.
-
Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC).Lung Cancer. 2019 Nov;137:113-122. doi: 10.1016/j.lungcan.2019.09.017. Epub 2019 Sep 23. Lung Cancer. 2019. PMID: 31568888 Free PMC article. Review.
-
Afatinib is especially effective against non-small cell lung cancer carrying an EGFR exon 19 deletion.Anticancer Res. 2015 Apr;35(4):2005-8. Anticancer Res. 2015. PMID: 25862853
-
Efficacy and safety of afatinib in Chinese patients with EGFR-mutated metastatic non-small-cell lung cancer (NSCLC) previously responsive to first-generation tyrosine-kinase inhibitors (TKI) and chemotherapy: comparison with historical cohort using erlotinib.BMC Cancer. 2016 Feb 24;16:147. doi: 10.1186/s12885-016-2201-9. BMC Cancer. 2016. PMID: 26911310 Free PMC article. Clinical Trial.
-
Dacomitinib in lung cancer: a "lost generation" EGFR tyrosine-kinase inhibitor from a bygone era?Drug Des Devel Ther. 2015 Oct 15;9:5641-53. doi: 10.2147/DDDT.S52787. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26508839 Free PMC article. Review.
Cited by
-
The utilization of next-generation sequencing to detect somatic mutations and predict clinical prognosis of Chinese non-small cell lung cancer patients.Onco Targets Ther. 2018 May 8;11:2637-2646. doi: 10.2147/OTT.S155995. eCollection 2018. Onco Targets Ther. 2018. PMID: 29780256 Free PMC article.
-
A Phase IIIb Open-Label, Single-Arm Study of Afatinib in EGFR TKI-Naïve Patients with EGFRm+ NSCLC: Final Analysis, with a Focus on Patients Enrolled at Sites in China.Target Oncol. 2022 Jan;17(1):1-13. doi: 10.1007/s11523-021-00859-6. Epub 2022 Jan 12. Target Oncol. 2022. PMID: 35020119 Free PMC article. Clinical Trial.
-
Overexpression of KRT17 promotes proliferation and invasion of non-small cell lung cancer and indicates poor prognosis.Cancer Manag Res. 2019 Aug 7;11:7485-7497. doi: 10.2147/CMAR.S218926. eCollection 2019. Cancer Manag Res. 2019. PMID: 31496806 Free PMC article.
-
Up-Regulation of MELK Promotes Cell Growth and Invasion by Accelerating G1/S Transition and Indicates Poor Prognosis in Lung Adenocarcinoma.Mol Biotechnol. 2024 Apr 27. doi: 10.1007/s12033-024-01143-4. Online ahead of print. Mol Biotechnol. 2024. PMID: 38676754
-
Melatonin enhances radiofrequency-induced NK antitumor immunity, causing cancer metabolism reprogramming and inhibition of multiple pulmonary tumor development.Signal Transduct Target Ther. 2021 Sep 1;6(1):330. doi: 10.1038/s41392-021-00745-7. Signal Transduct Target Ther. 2021. PMID: 34471091 Free PMC article. Clinical Trial.
References
-
- Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(4):504–535. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. - PubMed
-
- Travis WD, Brambilla E, Nicholson AG, et al. WHO Panel The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–1260. - PubMed
-
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. - PubMed
-
- Datta D, Lahiri B. Preoperative evaluation of patients undergoing lung resection surgery. Chest. 2003;123(6):2096–2103. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

