Platelet procoagulant phenotype is modulated by a p38-MK2 axis that regulates RTN4/Nogo proximal to the endoplasmic reticulum: utility of pathway analysis
- PMID: 29412690
- PMCID: PMC6008067
- DOI: 10.1152/ajpcell.00177.2017
Platelet procoagulant phenotype is modulated by a p38-MK2 axis that regulates RTN4/Nogo proximal to the endoplasmic reticulum: utility of pathway analysis
Abstract
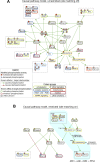
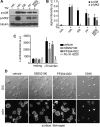
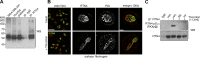
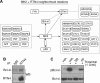
Upon encountering physiological cues associated with damaged or inflamed endothelium, blood platelets set forth intracellular responses to ultimately support hemostatic plug formation and vascular repair. To gain insights into the molecular events underlying platelet function, we used a combination of interactome, pathway analysis, and other systems biology tools to analyze associations among proteins functionally modified by reversible phosphorylation upon platelet activation. While an interaction analysis mapped out a relative organization of intracellular mediators in platelet signaling, pathway analysis revealed directional signaling relations around protein kinase C (PKC) isoforms and mitogen-activated protein kinases (MAPKs) associated with platelet cytoskeletal dynamics, inflammatory responses, and hemostatic function. Pathway and causality analysis further suggested that platelets activate a specific p38-MK2 axis to phosphorylate RTN4 (reticulon-4, also known as Nogo), a Bcl-xl sequestration protein and critical regulator of endoplasmic reticulum (ER) physiology. In vitro, we find that platelets drive a p38-MK2-RTN4-Bcl-xl pathway associated with the regulation of the ER and platelet phosphatidylserine exposure. Together, our results support the use of pathway tools in the analysis of omics data sets as a means to help generate novel, mechanistic, and testable hypotheses for platelet studies while uncovering RTN4 as a putative regulator of platelet cell physiological responses.
Keywords: Bcl-xl; CausalPath; MAPKAPK2; Pathway Commons; platelets.
Figures


Similar articles
-
Cell fate regulation by reticulon-4 in human prostate cancers.J Cell Physiol. 2019 Jul;234(7):10372-10385. doi: 10.1002/jcp.27704. Epub 2018 Nov 27. J Cell Physiol. 2019. PMID: 30480803
-
Platelet-Specific p38α Deficiency Improved Cardiac Function After Myocardial Infarction in Mice.Arterioscler Thromb Vasc Biol. 2017 Dec;37(12):e185-e196. doi: 10.1161/ATVBAHA.117.309856. Epub 2017 Oct 5. Arterioscler Thromb Vasc Biol. 2017. PMID: 28982666
-
Endoplasmic reticulum-associated ubiquitin-conjugating enzyme Ube2j1 is a novel substrate of MK2 (MAPKAP kinase-2) involved in MK2-mediated TNFα production.Biochem J. 2013 Dec 1;456(2):163-72. doi: 10.1042/BJ20130755. Biochem J. 2013. PMID: 24020373
-
Mitogen-activated protein kinase p38 and MK2, MK3 and MK5: ménage à trois or ménage à quatre?Cell Signal. 2010 Aug;22(8):1185-92. doi: 10.1016/j.cellsig.2010.03.002. Epub 2010 Mar 11. Cell Signal. 2010. PMID: 20227494 Review.
-
Novel Therapeutic Potential of Mitogen-Activated Protein Kinase Activated Protein Kinase 2 (MK2) in Chronic Airway Inflammatory Disorders.Curr Drug Targets. 2019;20(4):367-379. doi: 10.2174/1389450119666180816121323. Curr Drug Targets. 2019. PMID: 30112991 Review.
Cited by
-
Rho GTPase regulation of reactive oxygen species generation and signalling in platelet function and disease.Small GTPases. 2021 Sep-Nov;12(5-6):440-457. doi: 10.1080/21541248.2021.1878001. Epub 2021 Apr 12. Small GTPases. 2021. PMID: 33459160 Free PMC article. Review.
-
Large-scale automated machine reading discovers new cancer-driving mechanisms.Database (Oxford). 2018 Jan 1;2018:bay098. doi: 10.1093/database/bay098. Database (Oxford). 2018. PMID: 30256986 Free PMC article.
-
Hepatic kynurenic acid mediates phosphorylation of Nogo-A in the medial prefrontal cortex to regulate chronic stress-induced anxiety-like behaviors in mice.Acta Pharmacol Sin. 2024 Oct;45(10):2032-2044. doi: 10.1038/s41401-024-01302-y. Epub 2024 May 29. Acta Pharmacol Sin. 2024. PMID: 38811774
-
Unfolded Protein Response Differentially Modulates the Platelet Phenotype.Circ Res. 2022 Aug 5;131(4):290-307. doi: 10.1161/CIRCRESAHA.121.320530. Epub 2022 Jul 18. Circ Res. 2022. PMID: 35862006 Free PMC article.
-
The Toll-Like Receptor 2 Ligand Pam2CSK4 Activates Platelet Nuclear Factor-κB and Bruton's Tyrosine Kinase Signaling to Promote Platelet-Endothelial Cell Interactions.Front Immunol. 2021 Aug 30;12:729951. doi: 10.3389/fimmu.2021.729951. eCollection 2021. Front Immunol. 2021. PMID: 34527000 Free PMC article.
References
-
- Aslan JE. Platelet shape change. In: Platelets in Thrombotic and Non-Thrombotic Disorders, edited by Gresele P, López J, Kleiman N, Page C. New York: Springer, 2017. doi:10.1007/978-3-319-47462-5_24. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

