Neuronal Lipid Metabolism: Multiple Pathways Driving Functional Outcomes in Health and Disease
- PMID: 29410613
- PMCID: PMC5787076
- DOI: 10.3389/fnmol.2018.00010
Neuronal Lipid Metabolism: Multiple Pathways Driving Functional Outcomes in Health and Disease
Abstract
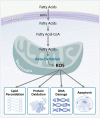
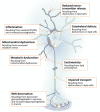
Lipids are a fundamental class of organic molecules implicated in a wide range of biological processes related to their structural diversity, and based on this can be broadly classified into five categories; fatty acids, triacylglycerols (TAGs), phospholipids, sterol lipids and sphingolipids. Different lipid classes play major roles in neuronal cell populations; they can be used as energy substrates, act as building blocks for cellular structural machinery, serve as bioactive molecules, or a combination of each. In amyotrophic lateral sclerosis (ALS), dysfunctions in lipid metabolism and function have been identified as potential drivers of pathogenesis. In particular, aberrant lipid metabolism is proposed to underlie denervation of neuromuscular junctions, mitochondrial dysfunction, excitotoxicity, impaired neuronal transport, cytoskeletal defects, inflammation and reduced neurotransmitter release. Here we review current knowledge of the roles of lipid metabolism and function in the CNS and discuss how modulating these pathways may offer novel therapeutic options for treating ALS.
Keywords: amyotrophic lateral sclerosis; glycosphingolipid; lipid metabolism; mitochondria; neuronal metabolism.
Figures

Similar articles
-
The role of lipids in the central nervous system and their pathological implications in amyotrophic lateral sclerosis.Semin Cell Dev Biol. 2021 Apr;112:69-81. doi: 10.1016/j.semcdb.2020.08.012. Epub 2020 Sep 19. Semin Cell Dev Biol. 2021. PMID: 32962914 Review.
-
Mitochondrial dysfunction is a converging point of multiple pathological pathways in amyotrophic lateral sclerosis.J Alzheimers Dis. 2010;20 Suppl 2:S311-24. doi: 10.3233/JAD-2010-100366. J Alzheimers Dis. 2010. PMID: 20463400 Review.
-
Mammalian lipids: structure, synthesis and function.Essays Biochem. 2021 Nov 2;65(5):813-845. doi: 10.1042/EBC20200067. Essays Biochem. 2021. PMID: 34415021 Free PMC article. Review.
-
Lipid Biosynthesis as an Antifungal Target.J Fungi (Basel). 2018 Apr 20;4(2):50. doi: 10.3390/jof4020050. J Fungi (Basel). 2018. PMID: 29677130 Free PMC article. Review.
-
AMPK Signalling and Defective Energy Metabolism in Amyotrophic Lateral Sclerosis.Neurochem Res. 2016 Mar;41(3):544-53. doi: 10.1007/s11064-015-1665-3. Epub 2015 Jul 23. Neurochem Res. 2016. PMID: 26202426 Review.
Cited by
-
Loss of heat shock factor initiates intracellular lipid surveillance by actin destabilization.Cell Rep. 2022 Oct 18;41(3):111493. doi: 10.1016/j.celrep.2022.111493. Cell Rep. 2022. PMID: 36261024 Free PMC article.
-
Downregulating carnitine palmitoyl transferase 1 affects disease progression in the SOD1 G93A mouse model of ALS.Commun Biol. 2021 Apr 30;4(1):509. doi: 10.1038/s42003-021-02034-z. Commun Biol. 2021. PMID: 33931719 Free PMC article.
-
Macroautophagy in CNS health and disease.Nat Rev Neurosci. 2022 Jul;23(7):411-427. doi: 10.1038/s41583-022-00588-3. Epub 2022 May 3. Nat Rev Neurosci. 2022. PMID: 35505254 Free PMC article. Review.
-
The Role of Lipids in Parkinson's Disease.Cells. 2019 Jan 7;8(1):27. doi: 10.3390/cells8010027. Cells. 2019. PMID: 30621069 Free PMC article. Review.
-
The lipid composition of extracellular vesicles: applications in diagnostics and therapeutic delivery.Front Mol Biosci. 2023 Jul 13;10:1198044. doi: 10.3389/fmolb.2023.1198044. eCollection 2023. Front Mol Biosci. 2023. PMID: 37520326 Free PMC article. Review.
References
-
- Agostoni C., Bruzzese M. G. (1992). Fatty acids: their biochemical and functional classification. Pediatr. Med. Chir. 14, 473–479. - PubMed
-
- Alexson S. E., Cannon B. (1984). A direct comparison between peroxisomal and mitochondrial preferences for fatty-acyl β-oxidation predicts channelling of medium-chain and very-long-chain unsaturated fatty acids to peroxisomes. Biochim. Biophys. Acta 796, 1–10. 10.1016/0005-2760(84)90231-5 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

