Diverse actions of the modulatory peptide neurotensin on central synaptic transmission
- PMID: 29405480
- PMCID: PMC6078827
- DOI: 10.1111/ejn.13858
Diverse actions of the modulatory peptide neurotensin on central synaptic transmission
Abstract
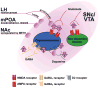
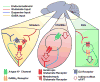
Neurotensin (NT) is a 13 amino acid neuropeptide that is expressed throughout the central nervous system and is implicated in the etiology of multiple diseases and disorders. Many primary investigations of NT-induced modulation of neuronal excitability at the level of the synapse have been conducted, but they have not been summarized in review form in nearly 30 years. Therefore, the goal of this review is to discuss the many actions of NT on neuronal excitability across brain regions as well as NT circuit architecture. In the basal ganglia as well as other brain nuclei, NT can act through diverse intracellular signaling cascades to enhance or depress neuronal activity by modulating activity of ion channels, ionotropic and metabotropic neurotransmitter receptors, and presynaptic release of neurotransmitters. Further, NT can produce indirect effects by evoking endocannabinoid release, and recently has itself been identified as a putative retrograde messenger. In the basal ganglia, the diverse actions and circuit architecture of NT signaling allow for input-specific control of reward-related behaviors.
Keywords: VTA; endocannabinoids; methamphetamine; neuropeptide; substantia nigra.
© 2018 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Figures
Similar articles
-
Neurotensin Induces Presynaptic Depression of D2 Dopamine Autoreceptor-Mediated Neurotransmission in Midbrain Dopaminergic Neurons.J Neurosci. 2015 Aug 5;35(31):11144-52. doi: 10.1523/JNEUROSCI.3816-14.2015. J Neurosci. 2015. PMID: 26245975 Free PMC article.
-
Neurotensin inhibits glutamate-mediated synaptic inputs onto ventral tegmental area dopamine neurons through the release of the endocannabinoid 2-AG.Neuropharmacology. 2012 Nov;63(6):983-91. doi: 10.1016/j.neuropharm.2012.07.037. Epub 2012 Jul 31. Neuropharmacology. 2012. PMID: 22884466
-
Neurotensin speeds inhibition of dopamine neurons through temporal modulation of GABAA and GABAB receptor-mediated synaptic input.Neuropharmacology. 2018 Mar 15;131:414-423. doi: 10.1016/j.neuropharm.2018.01.004. Epub 2018 Jan 5. Neuropharmacology. 2018. PMID: 29307543 Free PMC article.
-
The electrophysiological actions of neurotensin in the central nervous system.Life Sci. 1991;49(14):987-1002. doi: 10.1016/0024-3205(91)90300-z. Life Sci. 1991. PMID: 1890928 Review.
-
Neurotensin agonists as an alternative to antipsychotics.Expert Opin Investig Drugs. 2005 Apr;14(4):359-69. doi: 10.1517/13543784.14.4.359. Expert Opin Investig Drugs. 2005. PMID: 15882113 Review.
Cited by
-
Neurotensin: a neuropeptide induced by hCG in the human and rat ovary during the periovulatory period†.Biol Reprod. 2021 Jun 4;104(6):1337-1346. doi: 10.1093/biolre/ioab036. Biol Reprod. 2021. PMID: 33682882 Free PMC article.
-
Serum levels of neurotensin, pannexin-1, and sestrin-2 and the correlations with sleep quality or/and cognitive function in the patients with chronic insomnia disorder.Front Psychiatry. 2024 May 13;15:1360305. doi: 10.3389/fpsyt.2024.1360305. eCollection 2024. Front Psychiatry. 2024. PMID: 38803679 Free PMC article.
-
Dopamine D2 autoreceptor interactome: Targeting the receptor complex as a strategy for treatment of substance use disorder.Pharmacol Ther. 2020 Sep;213:107583. doi: 10.1016/j.pharmthera.2020.107583. Epub 2020 May 27. Pharmacol Ther. 2020. PMID: 32473160 Free PMC article. Review.
-
Analysis of the Underlying Mechanism of the Jiu Wei Zhen Xin Formula for Treating Generalized Anxiety Disorder Based on Network Pharmacology of Traditional Chinese Medicine.Evid Based Complement Alternat Med. 2022 May 29;2022:7761852. doi: 10.1155/2022/7761852. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35677384 Free PMC article.
-
Genome-Wide DNA Methylation Analysis in Male Methamphetamine Users With Different Addiction Qualities.Front Psychiatry. 2020 Oct 23;11:588229. doi: 10.3389/fpsyt.2020.588229. eCollection 2020. Front Psychiatry. 2020. PMID: 33192735 Free PMC article.
References
-
- Alexander MJ, Leeman SE. Widespread expression in adult rat forebrain of mRNA encoding high-affinity neurotensin receptor. J Comp Neurol. 1998;402:475–500. - PubMed
-
- Amano T, Wada E, Yamada D, Zushida K, Maeno H, Noda M, Wada K, Sekiguchi M. Heightened amygdala long-term potentiation in neurotensin receptor type-1 knockout mice. Neuropsychopharm. 2008;33:3135–3145. - PubMed
-
- Bayer VE, Towle AC, Pickel VM. Ultrastructural localization of neurotensin-like immunoreactivity within dense core vesicles in perikarya, but not terminals, colocalizing tyrosine hydroxylase in the rat ventral tegmental area. J Comp Neurol. 1991;311:179–196. - PubMed
-
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

