Chinmo prevents transformer alternative splicing to maintain male sex identity
- PMID: 29389999
- PMCID: PMC5811060
- DOI: 10.1371/journal.pgen.1007203
Chinmo prevents transformer alternative splicing to maintain male sex identity
Abstract
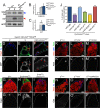
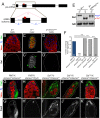
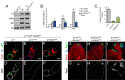
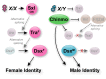
Reproduction in sexually dimorphic animals relies on successful gamete production, executed by the germline and aided by somatic support cells. Somatic sex identity in Drosophila is instructed by sex-specific isoforms of the DMRT1 ortholog Doublesex (Dsx). Female-specific expression of Sex-lethal (Sxl) causes alternative splicing of transformer (tra) to the female isoform traF. In turn, TraF alternatively splices dsx to the female isoform dsxF. Loss of the transcriptional repressor Chinmo in male somatic stem cells (CySCs) of the testis causes them to "feminize", resembling female somatic stem cells in the ovary. This somatic sex transformation causes a collapse of germline differentiation and male infertility. We demonstrate this feminization occurs by transcriptional and post-transcriptional regulation of traF. We find that chinmo-deficient CySCs upregulate tra mRNA as well as transcripts encoding tra-splice factors Virilizer (Vir) and Female lethal (2)d (Fl(2)d). traF splicing in chinmo-deficient CySCs leads to the production of DsxF at the expense of the male isoform DsxM, and both TraF and DsxF are required for CySC sex transformation. Surprisingly, CySC feminization upon loss of chinmo does not require Sxl but does require Vir and Fl(2)d. Consistent with this, we show that both Vir and Fl(2)d are required for tra alternative splicing in the female somatic gonad. Our work reveals the need for transcriptional regulation of tra in adult male stem cells and highlights a previously unobserved Sxl-independent mechanism of traF production in vivo. In sum, transcriptional control of the sex determination hierarchy by Chinmo is critical for sex maintenance in sexually dimorphic tissues and is vital in the preservation of fertility.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Chinmo is sufficient to induce male fate in somatic cells of the adult Drosophila ovary.Development. 2016 Mar 1;143(5):754-63. doi: 10.1242/dev.129627. Epub 2016 Jan 25. Development. 2016. PMID: 26811385 Free PMC article.
-
The Jak-STAT target Chinmo prevents sex transformation of adult stem cells in the Drosophila testis niche.Dev Cell. 2014 Nov 24;31(4):474-86. doi: 10.1016/j.devcel.2014.10.004. Epub 2014 Nov 13. Dev Cell. 2014. PMID: 25453558 Free PMC article.
-
The gene fl(2)d is needed for the sex-specific splicing of transformer pre-mRNA but not for double-sex pre-mRNA in Drosophila melanogaster.Mol Gen Genet. 1996 Nov 27;253(1-2):26-31. doi: 10.1007/s004380050292. Mol Gen Genet. 1996. PMID: 9003283
-
Masters change, slaves remain.Bioessays. 2003 Jan;25(1):1-4. doi: 10.1002/bies.10207. Bioessays. 2003. PMID: 12508274 Review.
-
Interplay between sex determination cascade and major signaling pathways during Drosophila eye development: Perspectives for future research.Dev Biol. 2021 Aug;476:41-52. doi: 10.1016/j.ydbio.2021.03.005. Epub 2021 Mar 18. Dev Biol. 2021. PMID: 33745943 Free PMC article. Review.
Cited by
-
Soma-germline communication drives sex maintenance in the Drosophila testis.Natl Sci Rev. 2024 Jun 22;11(8):nwae215. doi: 10.1093/nsr/nwae215. eCollection 2024 Aug. Natl Sci Rev. 2024. PMID: 39183747 Free PMC article.
-
Transcriptomic analysis of feminizing somatic stem cells in the Drosophila testis reveals putative downstream effectors of the transcription factor Chinmo.G3 (Bethesda). 2021 Apr 15;11(4):jkab067. doi: 10.1093/g3journal/jkab067. G3 (Bethesda). 2021. PMID: 33751104 Free PMC article.
-
SUMOylation of Jun fine-tunes the Drosophila gut immune response.PLoS Pathog. 2022 Mar 7;18(3):e1010356. doi: 10.1371/journal.ppat.1010356. eCollection 2022 Mar. PLoS Pathog. 2022. PMID: 35255103 Free PMC article.
-
Female-biased upregulation of insulin pathway activity mediates the sex difference in Drosophila body size plasticity.Elife. 2021 Jan 15;10:e58341. doi: 10.7554/eLife.58341. Elife. 2021. PMID: 33448263 Free PMC article.
-
Mamo decodes hierarchical temporal gradients into terminal neuronal fate.Elife. 2019 Sep 23;8:e48056. doi: 10.7554/eLife.48056. Elife. 2019. PMID: 31545163 Free PMC article.
References
-
- Lee G, Hall JC, Park JH. Doublesex gene expression in the central nervous system of Drosophila melanogaster. Journal of neurogenetics. 2002;16(4):229–48. . - PubMed
-
- Rideout EJ, Dornan AJ, Neville MC, Eadie S, Goodwin SF. Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nature neuroscience. 2010;13(4):458–66. doi: 10.1038/nn.2515 ; PubMed Central PMCID: PMC3092424. - DOI - PMC - PubMed
-
- Rideout EJ, Narsaiya MS, Grewal SS. The Sex Determination Gene transformer Regulates Male-Female Differences in Drosophila Body Size. PLoS genetics. 2015;11(12):e1005683 doi: 10.1371/journal.pgen.1005683 ; PubMed Central PMCID: PMC4692505. - DOI - PMC - PubMed
-
- Hudry B, Khadayate S, Miguel-Aliaga I. The sexual identity of adult intestinal stem cells controls organ size and plasticity. Nature. 2016;530(7590):344–8. doi: 10.1038/nature16953 ; PubMed Central PMCID: PMC4800002. - DOI - PMC - PubMed
-
- Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biology of sex differences. 2015;6:14 doi: 10.1186/s13293-015-0033-y ; PubMed Central PMCID: PMC4559072. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

