Identification and Biological Activity of Synthetic Macrophage Inducible C-Type Lectin Ligands
- PMID: 29387054
- PMCID: PMC5776103
- DOI: 10.3389/fimmu.2017.01940
Identification and Biological Activity of Synthetic Macrophage Inducible C-Type Lectin Ligands
Abstract
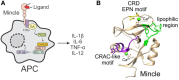
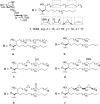
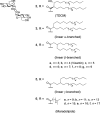
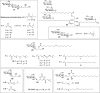
The macrophage inducible C-type lectin (Mincle) is a pattern recognition receptor able to recognize both damage-associated and pathogen-associated molecular patterns, and in this respect, there has been much interest in determining the scope of ligands that bind Mincle and how structural modifications to these ligands influence ensuing immune responses. In this review, we will present Mincle ligands of known chemical structure, with a focus on ligands that have been synthetically prepared, such as trehalose glycolipids, glycerol-based ligands, and 6-acylated glucose and mannose derivatives. The ability of the different classes of ligands to influence the innate, and consequently, the adaptive, immune response will be described, and where appropriate, structure-activity relationships within each class of Mincle ligands will be presented.
Keywords: C-type lectin; Mincle; adjuvant; damage-associated molecular pattern; glycolipid; pathogen-associated molecular pattern.
Figures




Similar articles
-
Mincle: 20 years of a versatile sensor of insults.Int Immunol. 2018 May 24;30(6):233-239. doi: 10.1093/intimm/dxy028. Int Immunol. 2018. PMID: 29726997 Review.
-
Synthesis and Biological Evaluation of Trehalose-based Bi-aryl Derivatives as C-type Lectin Ligands.Tetrahedron. 2023 Feb 13;132:133241. doi: 10.1016/j.tet.2022.133241. Epub 2023 Jan 9. Tetrahedron. 2023. PMID: 36874612 Free PMC article.
-
MCL and Mincle: C-Type Lectin Receptors That Sense Damaged Self and Pathogen-Associated Molecular Patterns.Front Immunol. 2014 Jun 23;5:288. doi: 10.3389/fimmu.2014.00288. eCollection 2014. Front Immunol. 2014. PMID: 25002863 Free PMC article. Review.
-
Structural analysis for glycolipid recognition by the C-type lectins Mincle and MCL.Proc Natl Acad Sci U S A. 2013 Oct 22;110(43):17438-43. doi: 10.1073/pnas.1312649110. Epub 2013 Oct 7. Proc Natl Acad Sci U S A. 2013. PMID: 24101491 Free PMC article.
-
Binding Sites for Acylated Trehalose Analogs of Glycolipid Ligands on an Extended Carbohydrate Recognition Domain of the Macrophage Receptor Mincle.J Biol Chem. 2016 Sep 30;291(40):21222-21233. doi: 10.1074/jbc.M116.749515. Epub 2016 Aug 19. J Biol Chem. 2016. PMID: 27542410 Free PMC article.
Cited by
-
The Role of Macrophage-Inducible C-Type Lectin in Different Stages of Chronic Liver Disease.Front Immunol. 2020 Jul 7;11:1352. doi: 10.3389/fimmu.2020.01352. eCollection 2020. Front Immunol. 2020. PMID: 32733451 Free PMC article.
-
6,6'-Aryl trehalose analogs as potential Mincle ligands.Bioorg Med Chem. 2020 Jul 15;28(14):115564. doi: 10.1016/j.bmc.2020.115564. Epub 2020 May 31. Bioorg Med Chem. 2020. PMID: 32616186 Free PMC article.
-
Design of Trehalose-Based Amide/Sulfonamide C-type Lectin Receptor Signaling Compounds.ChemMedChem. 2021 Apr 20;16(8):1246-1251. doi: 10.1002/cmdc.202000775. Epub 2021 Feb 9. ChemMedChem. 2021. PMID: 33415819 Free PMC article.
-
Expression and function of macrophage-inducible C-type lectin (Mincle) in inflammation driven parturition in fetal membranes and myometrium.Clin Exp Immunol. 2019 Jul;197(1):95-110. doi: 10.1111/cei.13281. Epub 2019 Mar 13. Clin Exp Immunol. 2019. PMID: 30793298 Free PMC article.
-
Synthesis of 12-O-Mono- and Diglycosyl-oxystearates, a New Class of Agonists for the C-type Lectin Receptor Mincle.ACS Med Chem Lett. 2018 Dec 13;10(1):44-49. doi: 10.1021/acsmedchemlett.8b00413. eCollection 2019 Jan 10. ACS Med Chem Lett. 2018. PMID: 30655945 Free PMC article.
References
-
- Matsumoto M, Tanaka T, Kaisho T, Sanjo H, Copeland NG, Gilbert DJ, et al. A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J Immunol (1999) 163:5039–48. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

