Fragile X Mental Retardation Protein Regulates Activity-Dependent Membrane Trafficking and Trans-Synaptic Signaling Mediating Synaptic Remodeling
- PMID: 29375303
- PMCID: PMC5770364
- DOI: 10.3389/fnmol.2017.00440
Fragile X Mental Retardation Protein Regulates Activity-Dependent Membrane Trafficking and Trans-Synaptic Signaling Mediating Synaptic Remodeling
Abstract
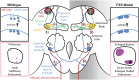
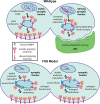
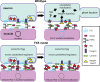
Fragile X syndrome (FXS) is the leading monogenic cause of autism and intellectual disability. The disease arises through loss of fragile X mental retardation protein (FMRP), which normally exhibits peak expression levels in early-use critical periods, and is required for activity-dependent synaptic remodeling during this transient developmental window. FMRP canonically binds mRNA to repress protein translation, with targets that regulate cytoskeleton dynamics, membrane trafficking, and trans-synaptic signaling. We focus here on recent advances emerging in these three areas from the Drosophila disease model. In the well-characterized central brain mushroom body (MB) olfactory learning/memory circuit, FMRP is required for activity-dependent synaptic remodeling of projection neurons innervating the MB calyx, with function tightly restricted to an early-use critical period. FMRP loss is phenocopied by conditional removal of FMRP only during this critical period, and rescued by FMRP conditional expression only during this critical period. Consistent with FXS hyperexcitation, FMRP loss defects are phenocopied by heightened sensory experience and targeted optogenetic hyperexcitation during this critical period. FMRP binds mRNA encoding Drosophila ESCRTIII core component Shrub (human CHMP4 homolog) to restrict Shrub translation in an activity-dependent mechanism only during this same critical period. Shrub mediates endosomal membrane trafficking, and perturbing Shrub expression is known to interfere with neuronal process pruning. Consistently, FMRP loss and Shrub overexpression targeted to projection neurons similarly causes endosomal membrane trafficking defects within synaptic boutons, and genetic reduction of Shrub strikingly rescues Drosophila FXS model defects. In parallel work on the well-characterized giant fiber (GF) circuit, FMRP limits iontophoretic dye loading into central interneurons, demonstrating an FMRP role controlling core neuronal properties through the activity-dependent repression of translation. In the well-characterized Drosophila neuromuscular junction (NMJ) model, developmental synaptogenesis and activity-dependent synaptic remodeling both require extracellular matrix metalloproteinase (MMP) enzymes interacting with the heparan sulfate proteoglycan (HSPG) glypican dally-like protein (Dlp) to restrict trans-synaptic Wnt signaling, with FXS synaptogenic defects alleviated by both MMP and HSPG reduction. This new mechanistic axis spanning from activity to FMRP to HSPG-dependent MMP regulation modulates activity-dependent synaptogenesis. We discuss future directions for these mechanisms, and intersecting research priorities for FMRP in glial and signaling interactions.
Keywords: Drosophila; critical period; fragile X syndrome; signaling; synapse.
Figures
Similar articles
-
Neuron-Specific FMRP Roles in Experience-Dependent Remodeling of Olfactory Brain Innervation during an Early-Life Critical Period.J Neurosci. 2021 Feb 10;41(6):1218-1241. doi: 10.1523/JNEUROSCI.2167-20.2020. Epub 2021 Jan 5. J Neurosci. 2021. PMID: 33402421 Free PMC article.
-
Fragile X Mental Retardation Protein positively regulates PKA anchor Rugose and PKA activity to control actin assembly in learning/memory circuitry.Neurobiol Dis. 2019 Jul;127:53-64. doi: 10.1016/j.nbd.2019.02.004. Epub 2019 Feb 13. Neurobiol Dis. 2019. PMID: 30771457 Free PMC article.
-
Neuronal activity drives FMRP- and HSPG-dependent matrix metalloproteinase function required for rapid synaptogenesis.Sci Signal. 2017 Nov 7;10(504):eaan3181. doi: 10.1126/scisignal.aan3181. Sci Signal. 2017. PMID: 29114039 Free PMC article.
-
Impaired activity-dependent neural circuit assembly and refinement in autism spectrum disorder genetic models.Front Cell Neurosci. 2014 Feb 7;8:30. doi: 10.3389/fncel.2014.00030. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24570656 Free PMC article. Review.
-
Multifarious Functions of the Fragile X Mental Retardation Protein.Trends Genet. 2017 Oct;33(10):703-714. doi: 10.1016/j.tig.2017.07.008. Epub 2017 Aug 18. Trends Genet. 2017. PMID: 28826631 Free PMC article. Review.
Cited by
-
From circuits to behavior: Amygdala dysfunction in fragile X syndrome.Front Integr Neurosci. 2023 Mar 9;17:1128529. doi: 10.3389/fnint.2023.1128529. eCollection 2023. Front Integr Neurosci. 2023. PMID: 36969493 Free PMC article. Review.
-
Activity-Dependent Remodeling of Drosophila Olfactory Sensory Neuron Brain Innervation during an Early-Life Critical Period.J Neurosci. 2019 Apr 17;39(16):2995-3012. doi: 10.1523/JNEUROSCI.2223-18.2019. Epub 2019 Feb 12. J Neurosci. 2019. PMID: 30755492 Free PMC article.
-
Developmental studies in fragile X syndrome.J Neurodev Disord. 2020 May 2;12(1):13. doi: 10.1186/s11689-020-09310-9. J Neurodev Disord. 2020. PMID: 32359368 Free PMC article. Review.
-
Drosophila motor neuron boutons remodel through membrane blebbing coupled with muscle contraction.Nat Commun. 2023 Jun 8;14(1):3352. doi: 10.1038/s41467-023-38421-9. Nat Commun. 2023. PMID: 37291089 Free PMC article.
-
Drosophila melanogaster as a Model to Study the Multiple Phenotypes, Related to Genome Stability of the Fragile-X Syndrome.Front Genet. 2019 Feb 13;10:10. doi: 10.3389/fgene.2019.00010. eCollection 2019. Front Genet. 2019. PMID: 30815010 Free PMC article. Review.
References
-
- Antar L. N., Afroz R., Dictenberg J. B., Carroll R. C., Bassell G. J. (2004). Metabotropic glutamate receptor activation regulates Fragile X mental retardation protein and FMR1 mRNA localization differentially in dendrites and at synapses. J. Neurosci. 24 2648–2655. 10.1523/JNEUROSCI.0099-04.2004 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

