Oviductal extracellular vesicles (oviductosomes, OVS) are conserved in humans: murine OVS play a pivotal role in sperm capacitation and fertility
- PMID: 29370405
- PMCID: PMC6454811
- DOI: 10.1093/molehr/gay003
Oviductal extracellular vesicles (oviductosomes, OVS) are conserved in humans: murine OVS play a pivotal role in sperm capacitation and fertility
Abstract
Study questions: Are extracellular vesicles (EVs) in the murine oviduct (oviductosomes, OVS) conserved in humans and do they play a role in the fertility of Pmca4-/- females?
Summary answer: OVS and their fertility-modulating proteins are conserved in humans, arise via the apocrine pathway, and mediate a compensatory upregulation of PMCA1 (plasma membrane Ca2+-ATPase 1) in Pmca4-/- female mice during proestrus/estrus, to account for their fertility.
What is known already: Recently murine OVS were identified and shown during proestrus/estrus to express elevated levels of PMCA4 which they can deliver to sperm. PMCA4 is the major Ca2+ efflux pump in murine sperm and Pmca4 deletion leads to loss of sperm motility and male infertility as there is no compensatory upregulation of the remaining Ca2+ pump, PMCA1. Of the four family members of PMCAs (PMCA1-4), PMCA1 and PMCA4 are ubiquitous, and to date there have been no reports of one isoform being upregulated to compensate for another in any organ/tissue. Since Pmca4-/- females are fertile, despite the abundant expression of PMCA4 in wild-type (WT) OVS, we propose that OVS serve a role of packaging and delivering to sperm elevated levels of PMCA1 in Pmca4-/- during proestrus/estrus to compensate for PMCA4's absence.
Study design, size, duration: Fallopian tubes from pre-menopausal women undergoing hysterectomy were used to study EVs in the luminal fluid. Oviducts from sexually mature WT mice were sectioned after perfusion fixation to detect EVs in situ. Oviducts were recovered from WT and Pmca4-/- after hormonally induced estrus and sectioned for PMCA1 immunofluorescence (IF) (detected with confocal microscopy) and hematoxylin and eosin staining. Reproductive tissues, luminal fluids and EVs were recovered after induced estrus and after natural cycling for western blot analysis of PMCA1 and qRT-PCR of Pmca1 to compare expression levels in WT and Pmca4-/-. OVS, uterosomes, and epididymal luminal fluid were included in the comparisons. WT and Pmca4-/- OVS were analyzed for the presence of known PMCA4 partners in sperm and their ability to interact with PMCA1, via co-immunoprecipitation. In vitro uptake of PMCA1 from OVS was analyzed in capacitated and uncapacitated sperm via quantitative western blot analysis, IF localization and flow cytometry. Caudal sperm were also assayed for uptake of tyrosine-phosphorylated proteins which were shown to be present in OVS. Finally, PMCA1 and PMCA4 in OVS and that delivered to sperm were assayed for enzymatic activity.
Participants/materials, setting, methods: Human fallopian tubes were flushed to recover luminal fluid which was processed for OVS via ultracentrifugation. Human OVS were negatively stained for transmission electron microscopy (TEM) and subjected to immunogold labeling, to detect PMCA4. Western analysis was used to detect HSC70 (an EV biomarker), PMCA1 and endothelial nitric oxide synthase (eNOS) which is a fertility-modulating protein delivered to human sperm by prostasomes. Oviducts of sexually mature female mice were sectioned after perfusion fixation for TEM tomography to obtain 3D information and to distinguish cross-sections of EVs from those of microvilli and cilia. Murine tissues, luminal fluids and EVs were assayed for PMCA1 (IF and western blot) or qRT-PCR. PMCA1 levels from western blots were quantified, using band densities and compared in WT and Pmca4-/- after induced estrus and in proestrus/estrus and metestrus/diestrus in cycling females. In vitro uptake of PMCA1 and tyrosine-phosphorylated proteins was quantified with flow cytometry and/or quantitative western blot. Ca2+-ATPase activity in OVS and sperm before and after PMCA1 and PMCA4 uptake was assayed, via the enzymatic hydrolysis rate of ATP.
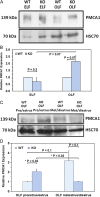
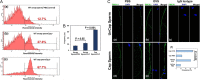
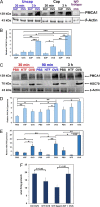
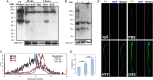
Main results and the role of chance: TEM revealed that human oviducts contain EVs (exosomal and microvesicular). These EVs contain PMCA4 (immunolabeling), eNOS and PMCA1 (western blot) in their cargo. TEM tomography showed the murine oviduct with EV-containing blebs which typify the apocrine pathway for EV biogenesis. Western blots revealed that during proestrus/estrus PMCA1 was significantly elevated in the oviductal luminal fluid (OLF) (P = 0.02) and in OVS (P = 0.03) of Pmca4-/-, compared to WT. Further, while PMCA1 levels did not fluctuate in OLF during the cycle in WT, they were significantly (P = 0.02) higher in proestrus/estrus than at metestrus/diestrus in Pmca4-/-. The elevated levels of PMCA1 in proestrus/estrus, which mimics PMCA4 in WT, is OLF/OVS-specific, and is not seen in oviductal tissues, uterosomes or epididymal luminal fluid of Pmca4-/-. However, qRT-PCR revealed significantly elevated levels of Pmca1 transcript in Pmca4-/- oviductal tissues, compared to WT. PMCA1 could be transferred from OVS to sperm and the levels were significantly higher for capacitated vs uncapacitated sperm, as assessed by flow cytometry (P = 0.001) after 3 h co-incubation, quantitative western blot (P < 0.05) and the frequency of immuno-labeled sperm (P < 0.001) after 30 min co-incubation. Tyrosine phosphorylated proteins were discovered in murine OVS and could be delivered to sperm after their co-incubation with OVS, as detected by western, immunofluorescence localization, and flow cytometry. PMCA1 and PMCA4 in OVS were shown to be enzymatically active and this activity increased in sperm after OVS interaction.
Large scale data: None.
Limitations reasons for caution: Although oviductal tissues of WT and Pmca4-/- showed no significant difference in PMCA1 levels, Pmca4-/- levels of OVS/OLF during proestrus/estrus were significantly higher than in WT. We have attributed this enrichment or upregulation of PMCA1 in Pmca4-/- partly to selective packaging in OVS to compensate for the lack of PMCA4. However, in the absence of a difference between WT and Pmca4-/- in the PMCA1 levels in oviductal tissues as a whole, we cannot rule out significantly higher PMCA1 expression in the oviductal epithelium that gives rise to the OVS as significantly higher Pmca1 transcripts were detected in Pmca4-/-.
Wider implications of the findings: Since OVS and fertility-modulating cargo components are conserved in humans, it suggests that murine OVS role in regulating the expression of proteins required for capacitation and fertility is also conserved. Secondly, OVS may explain some of the differences in in vivo and in vitro fertilization for mouse mutants, as seen in mice lacking the gene for FER which is the enzyme required for sperm protein tyrosine phosphorylation. Our observation that murine OVS carry and can modulate sperm protein tyrosine phosphorylation by delivering them to sperm provides an explanation for the in vivo fertility of Fer mutants, not seen in vitro. Finally, our findings have implications for infertility treatment and exosome therapeutics.
Study funding and competing interest(s): The work was supported by National Institute of Health (RO3HD073523 and 5P20RR015588) grants to P.A.M.-D. There are no conflicts of interests.
Figures





Similar articles
-
Junctional adhesion molecule A: expression in the murine epididymal tract and accessory organs and acquisition by maturing sperm.Mol Hum Reprod. 2017 Feb 10;23(2):132-140. doi: 10.1093/molehr/gaw082. Mol Hum Reprod. 2017. PMID: 28062807 Free PMC article.
-
Expression and secretion of plasma membrane Ca2+-ATPase 4a (PMCA4a) during murine estrus: association with oviductal exosomes and uptake in sperm.PLoS One. 2013 Nov 14;8(11):e80181. doi: 10.1371/journal.pone.0080181. eCollection 2013. PLoS One. 2013. PMID: 24244642 Free PMC article.
-
Plasma membrane Ca2+-ATPase 4: interaction with constitutive nitric oxide synthases in human sperm and prostasomes which carry Ca2+/CaM-dependent serine kinase.Mol Hum Reprod. 2015 Nov;21(11):832-43. doi: 10.1093/molehr/gav049. Epub 2015 Sep 7. Mol Hum Reprod. 2015. PMID: 26345709 Free PMC article.
-
Role of exosomes in the reproductive tract Oviductosomes mediate interactions of oviductal secretion with gametes/early embryo.Front Biosci (Landmark Ed). 2016 Jun 1;21(6):1278-85. doi: 10.2741/4456. Front Biosci (Landmark Ed). 2016. PMID: 27100506 Review.
-
Uterosomes: Exosomal cargo during the estrus cycle and interaction with sperm.Front Biosci (Schol Ed). 2016 Jan 1;8(1):115-22. doi: 10.2741/s451. Front Biosci (Schol Ed). 2016. PMID: 26709901 Review.
Cited by
-
Oviductal Extracellular Vesicles Improve Post-Thaw Sperm Function in Red Wolves and Cheetahs.Int J Mol Sci. 2020 May 25;21(10):3733. doi: 10.3390/ijms21103733. Int J Mol Sci. 2020. PMID: 32466321 Free PMC article.
-
The Roles of Extracellular Vesicles and Organoid Models in Female Reproductive Physiology.Int J Mol Sci. 2022 Mar 16;23(6):3186. doi: 10.3390/ijms23063186. Int J Mol Sci. 2022. PMID: 35328607 Free PMC article. Review.
-
The Role of Thyroid Hormones in Hepatocyte Proliferation and Liver Cancer.Front Endocrinol (Lausanne). 2019 Aug 30;10:532. doi: 10.3389/fendo.2019.00532. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31543862 Free PMC article. Review.
-
Extracellular vesicles and melatonin benefit embryonic develop by regulating reactive oxygen species and 5-methylcytosine.J Pineal Res. 2020 Apr;68(3):e12635. doi: 10.1111/jpi.12635. Epub 2020 Feb 16. J Pineal Res. 2020. PMID: 32012354 Free PMC article.
-
Deciphering the oviductal extracellular vesicles content across the estrous cycle: implications for the gametes-oviduct interactions and the environment of the potential embryo.BMC Genomics. 2018 Aug 22;19(1):622. doi: 10.1186/s12864-018-4982-5. BMC Genomics. 2018. PMID: 30134841 Free PMC article.
References
-
- Aalberts M, van Dissel-Emiliani FM, van Adrichem NP, van Wijnen M, Wauben MH, Stout TA, Stoorvogel W. Identification of distinct populations of prostasomes that differentially express prostate stem cell antigen, annexin A1, and GLIPR2 in humans. Biol Reprod 2012;86:82. - PubMed
-
- Adeoya-Osiguwa SA, Fraser LR. Evidence for Ca2+-dependent ATPase activity, stimulated by decapacitation factor and calmodulin, in mouse sperm. Mol Reprod Dev 1996;44:111–120. - PubMed
-
- Alvau A, Battistone MA, Gervasi MG, Navarrete FA, Xu X, Sanchez-Cardenas C, De la Vega-Beltran JL, Da Ros VG, Greer PA, Darszon A et al. . The tyrosine kinase FER is responsible for the capacitation-associated increase in tyrosine phosphorylation in murine sperm. Development 2016;143:2325–2333. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

