CCR2 upregulation in DRG neurons plays a crucial role in gastric hyperalgesia associated with diabetic gastropathy
- PMID: 29359616
- PMCID: PMC5784547
- DOI: 10.1177/1744806917751322
CCR2 upregulation in DRG neurons plays a crucial role in gastric hyperalgesia associated with diabetic gastropathy
Abstract
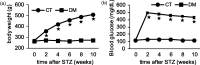
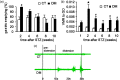
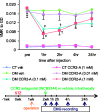
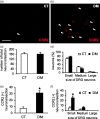
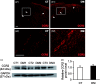
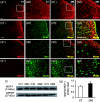
Background Diabetic gastropathy is a complex neuromuscular dysfunction of the stomach that commonly occurs in diabetes mellitus. Diabetic patients often present with upper gastrointestinal symptoms, such as epigastric discomfort or pain. The aim of this study was to assess gastric sensation in streptozocin-induced diabetes mellitus (DM) rats and to determine the contribution of C-C motif chemokine receptor 2 (CCR2) signaling to gastric hyperalgesia. Results DM rats showed signs of neuropathy (cutaneous mechanical hyperalgesia) from two weeks after streptozocin administration until the end of the experiment. Accelerated solid gastric emptying was observed at two weeks after streptozocin administration compared to the controls. Intense gastric hyperalgesia also developed in DM rats at two weeks after streptozocin administration, which was significantly reduced after intrathecal administration of the CCR2 antagonist INCB3344. Immunochemical analysis indicated that CCR2 expression was substantially upregulated in small and medium-sized dorsal root ganglia neurons of DM rats, although the protein level of monocyte chemoattractant protein-1, the preferred ligand for CCR2, was not significantly different between the control and DM groups. Conclusions These data suggest that CCR2 activation in nociceptive dorsal root ganglia neurons plays a role in the pathogenesis of gastric hyperalgesia associated with diabetic gastropathy and that CCR2 antagonist may be a promising treatment for therapeutic intervention.
Keywords: CCR2 receptor; Neuropathic pain; diabetes mellitus; gastric hyperalgesia; streptozocin.
Figures


Similar articles
-
Contribution of chemokine CCL2/CCR2 signaling in the dorsal root ganglion and spinal cord to the maintenance of neuropathic pain in a rat model of lumbar disc herniation.J Pain. 2014 May;15(5):516-26. doi: 10.1016/j.jpain.2014.01.492. Epub 2014 Jan 23. J Pain. 2014. PMID: 24462503
-
Mechanistic insights into the role of the chemokine CCL2/CCR2 axis in dorsal root ganglia to peripheral inflammation and pain hypersensitivity.J Neuroinflammation. 2021 Mar 23;18(1):79. doi: 10.1186/s12974-021-02125-y. J Neuroinflammation. 2021. PMID: 33757529 Free PMC article.
-
Diabetes-induced Neuropathic Mechanical Hyperalgesia Depends on P2X4 Receptor Activation in Dorsal Root Ganglia.Neuroscience. 2019 Feb 1;398:158-170. doi: 10.1016/j.neuroscience.2018.12.003. Epub 2018 Dec 8. Neuroscience. 2019. PMID: 30537520
-
Chemokine signaling and the management of neuropathic pain.Mol Interv. 2009 Aug;9(4):188-95. doi: 10.1124/mi.9.4.7. Mol Interv. 2009. PMID: 19720751 Free PMC article. Review.
-
Diabetic gastropathy: gastric neuromuscular dysfunction in diabetes mellitus: a review of symptoms, pathophysiology, and treatment.Dig Dis Sci. 1999 Jun;44(6):1061-75. doi: 10.1023/a:1026647417465. Dig Dis Sci. 1999. PMID: 10389675 Review.
Cited by
-
A Missing Puzzle in Preclinical Studies-Are CCR2, CCR5, and Their Ligands' Roles Similar in Obesity-Induced Hypersensitivity and Diabetic Neuropathy?-Evidence from Rodent Models and Clinical Studies.Int J Mol Sci. 2024 Oct 21;25(20):11323. doi: 10.3390/ijms252011323. Int J Mol Sci. 2024. PMID: 39457105 Free PMC article. Review.
-
Motor Dysfunction of Gastric Antral Smooth Muscle in Diabetic Rats: Contribution of ATP-Dependent Potassium Channels.Adv Biomed Res. 2023 Jul 27;12:199. doi: 10.4103/abr.abr_44_23. eCollection 2023. Adv Biomed Res. 2023. PMID: 37694236 Free PMC article.
-
Overexpression of Purinergic P2X4 Receptors in Hippocampus Rescues Memory Impairment in Rats with Type 2 Diabetes.Neurosci Bull. 2020 Jul;36(7):719-732. doi: 10.1007/s12264-020-00478-7. Epub 2020 Mar 20. Neurosci Bull. 2020. PMID: 32198702 Free PMC article.
-
Downregulation of glucose-6-phosphate dehydrogenase contributes to diabetic neuropathic pain through upregulation of toll-like receptor 4 in rats.Mol Pain. 2019 Jan-Dec;15:1744806919838659. doi: 10.1177/1744806919838659. Mol Pain. 2019. PMID: 30838902 Free PMC article.
-
A New Application for Cenicriviroc, a Dual CCR2/CCR5 Antagonist, in the Treatment of Painful Diabetic Neuropathy in a Mouse Model.Int J Mol Sci. 2024 Jul 5;25(13):7410. doi: 10.3390/ijms25137410. Int J Mol Sci. 2024. PMID: 39000516 Free PMC article.
References
-
- International Diabetes Federation. IDF Diabetes Atlas, 7th ed. 2015, https://www.idf.org/e-library/epidemiology-research/diabetes-atlas.html
-
- Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005; 28: 956–962. - PubMed
-
- Feldman EL. Epidemiology and classification of diabetic neuropathy – up to date, https://www.uptodate.com (2015, accessed 5 January 2017).
-
- Vinik AI, Maser RE, Mitchell BD, et al. Diabetic autonomic neuropathy. Diabetes Care 2003; 26: 1553–1579. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

