MOVING: Motivation-Oriented interVention study for the elderly IN Greifswald: study protocol for a randomized controlled trial
- PMID: 29357943
- PMCID: PMC5778817
- DOI: 10.1186/s13063-017-2425-2
MOVING: Motivation-Oriented interVention study for the elderly IN Greifswald: study protocol for a randomized controlled trial
Abstract
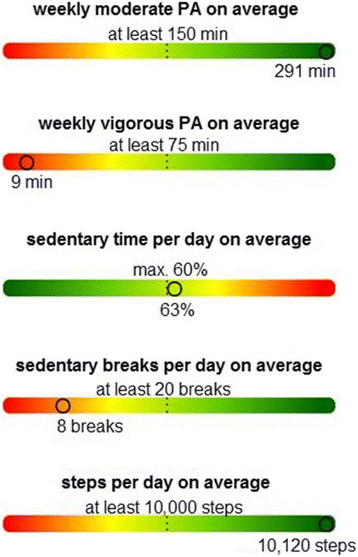
Background: Cardiovascular diseases (CVD) are the leading cause of mortality. In 2014, they were responsible for 38.9% of all causes of death in Germany. One major risk factor for CVD is a lack of physical activity (PA). A health-promoting lifestyle including regular PA and minimizing sitting time (ST) in daily life is a central preventive measure. Previous studies have shown that PA decreases in older age; 2.4-29% of the people aged over 60 years achieve the World Health Organization recommendations. This age group spends on average 9.4 h per day in sedentary activities. To increase PA and decrease ST, a low-threshold intervention, consisting of individualized feedback letters based on objectively measured data of PA and ST, was developed. The research question is: Do individual feedback letters, based on accelerometer data, have a positive effect on PA and ST?
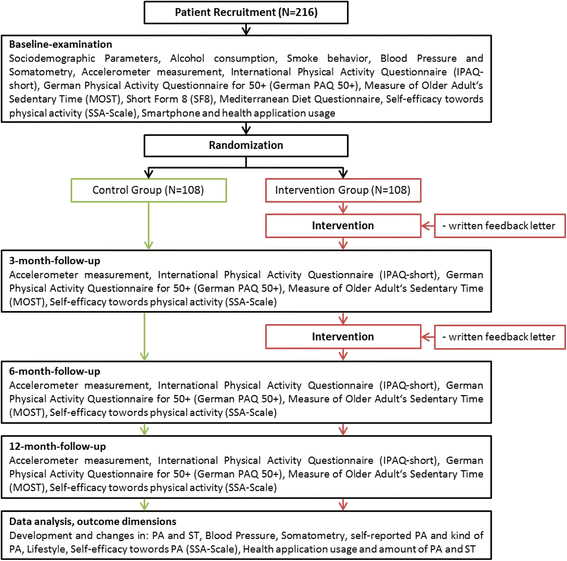
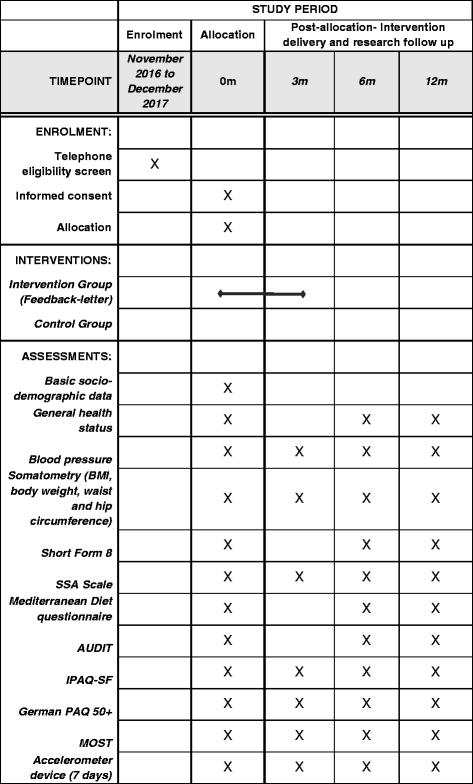
Methods/design: MOVING is a two-arm, randomized controlled trial. Inclusion criteria are age ≥ 65 years and the ability to be physically active. Exclusion criteria are the permanent use of a wheelchair and simultaneous participation in another study on PA. At baseline participants who give informed consent will receive general information and recommendations about the positive effects of regular PA and less ST. Participants of both groups will receive an accelerometer device, which records PA and ST over a period of seven consecutive days following by a randomization. Participants in the intervention group will receive automatically generated, individualized feedback letters by mail based on their PA and ST at baseline and at 3-month follow-up. Further follow-up examinations will be carried out at 6 and 12 months. The primary outcome is the increase of PA and the reduction of ST after 6 months in the intervention group compared to the control group.
Discussion: The goal of the study is to examine the effects of a simple feedback intervention on PA and ST in elderly people. We aim to achieve an effect of 20% increase in moderate-to-vigorous physical activity (MVPA). The intervention may have the potential to decrease crucial cardiovascular risk factors and, therefore, contribute to prevention of CVD.
Trial registration: German Clinical Trials Register, ID: DRKS00010410 . Registered on 17 May 2017.
Keywords: Accelerometry; Behavior change; Elderly individuals (people); Intervention; Physical activity; RCT; Sedentary behavior; motivation.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the University Medicine Greifswald (ethic approval BB071/16).
All participants have to provide their written informed consent before they can participate in the study. The original declaration remains in the examination center, participants will receive a copy. Study participants can withdraw their consent any time without negative consequences for them.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
A low-threshold intervention to increase physical activity and reduce physical inactivity in a group of healthy elderly people in Germany: Results of the randomized controlled MOVING study.PLoS One. 2021 Sep 16;16(9):e0257326. doi: 10.1371/journal.pone.0257326. eCollection 2021. PLoS One. 2021. PMID: 34529735 Free PMC article. Clinical Trial.
-
Mobile Exergaming for Health-Effects of a serious game application for smartphones on physical activity and exercise adherence in type 2 diabetes mellitus-study protocol for a randomized controlled trial.Trials. 2017 Mar 6;18(1):103. doi: 10.1186/s13063-017-1853-3. Trials. 2017. PMID: 28264717 Free PMC article. Clinical Trial.
-
Unravelling effectiveness of a nurse-led behaviour change intervention to enhance physical activity in patients at risk for cardiovascular disease in primary care: study protocol for a cluster randomised controlled trial.Trials. 2017 Feb 22;18(1):79. doi: 10.1186/s13063-017-1823-9. Trials. 2017. PMID: 28228151 Free PMC article. Clinical Trial.
-
Behavioral Counseling to Promote a Healthful Diet and Physical Activity for Cardiovascular Disease Prevention in Adults Without Known Cardiovascular Disease Risk Factors: Updated Systematic Review for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2017 Jul. Report No.: 15-05222-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2017 Jul. Report No.: 15-05222-EF-1. PMID: 29364620 Free Books & Documents. Review.
-
Utility of Consumer Physical Activity Trackers as an Intervention Tool in Cardiovascular Disease Prevention and Treatment.Prog Cardiovasc Dis. 2016 May-Jun;58(6):613-9. doi: 10.1016/j.pcad.2016.02.006. Epub 2016 Mar 2. Prog Cardiovasc Dis. 2016. PMID: 26943981 Review.
Cited by
-
Interventions for reducing sedentary behaviour in community-dwelling older adults.Cochrane Database Syst Rev. 2021 Jun 25;6(6):CD012784. doi: 10.1002/14651858.CD012784.pub2. Cochrane Database Syst Rev. 2021. PMID: 34169503 Free PMC article.
-
Levels of and determinants for physical activity and physical inactivity in a group of healthy elderly people in Germany: Baseline results of the MOVING-study.PLoS One. 2020 Aug 13;15(8):e0237495. doi: 10.1371/journal.pone.0237495. eCollection 2020. PLoS One. 2020. PMID: 32790711 Free PMC article.
-
Design and methodology of the impact of HemoDiaFIlTration on physical activity and self-reported outcomes: a randomized controlled trial (HDFIT trial) in Brazil.BMC Nephrol. 2019 Mar 20;20(1):98. doi: 10.1186/s12882-019-1247-8. BMC Nephrol. 2019. PMID: 30894141 Free PMC article. Clinical Trial.
-
The Effectiveness of Interventions to Reduce Sedentary Time in Different Target Groups and Settings in Germany: Systematic Review, Meta-Analysis and Recommendations on Interventions.Int J Environ Res Public Health. 2022 Aug 17;19(16):10178. doi: 10.3390/ijerph191610178. Int J Environ Res Public Health. 2022. PMID: 36011821 Free PMC article. Review.
-
A low-threshold intervention to increase physical activity and reduce physical inactivity in a group of healthy elderly people in Germany: Results of the randomized controlled MOVING study.PLoS One. 2021 Sep 16;16(9):e0257326. doi: 10.1371/journal.pone.0257326. eCollection 2021. PLoS One. 2021. PMID: 34529735 Free PMC article. Clinical Trial.
References
-
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical