Inhibiting the Ins and Outs of HIV Replication: Cell-Intrinsic Antiretroviral Restrictions at the Plasma Membrane
- PMID: 29354117
- PMCID: PMC5758531
- DOI: 10.3389/fimmu.2017.01853
Inhibiting the Ins and Outs of HIV Replication: Cell-Intrinsic Antiretroviral Restrictions at the Plasma Membrane
Abstract
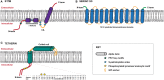
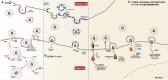
Like all viruses, human immunodeficiency viruses (HIVs) and their primate lentivirus relatives must enter cells in order to replicate and, once produced, new virions need to exit to spread to new targets. These processes require the virus to cross the plasma membrane of the cell twice: once via fusion mediated by the envelope glycoprotein to deliver the viral core into the cytosol; and secondly by ESCRT-mediated scission of budding virions during release. This physical barrier thus presents a perfect location for host antiviral restrictions that target enveloped viruses in general. In this review we will examine the current understanding of innate host antiviral defences that inhibit these essential replicative steps of primate lentiviruses associated with the plasma membrane, the mechanism by which these viruses have adapted to evade such defences, and the role that this virus/host battleground plays in the transmission and pathogenesis of HIV/AIDS.
Keywords: antiviral restriction; human immunodeficiency virus; interferon-induced transmembrane; plasma membrane; serine incorporator; tetherin/BST-2; type I interferons.
Figures

Similar articles
-
Antiviral activity of the interferon-induced cellular protein BST-2/tetherin.AIDS Res Hum Retroviruses. 2009 Dec;25(12):1197-210. doi: 10.1089/aid.2009.0253. AIDS Res Hum Retroviruses. 2009. PMID: 19929170 Free PMC article. Review.
-
Feline immunodeficiency virus envelope glycoproteins antagonize tetherin through a distinctive mechanism that requires virion incorporation.J Virol. 2014 Mar;88(6):3255-72. doi: 10.1128/JVI.03814-13. Epub 2014 Jan 3. J Virol. 2014. PMID: 24390322 Free PMC article.
-
Understanding restriction factors and intrinsic immunity: insights and lessons from the primate lentiviruses.Future Virol. 2014;9(5):483-497. doi: 10.2217/FVL.14.25. Future Virol. 2014. PMID: 26543491 Free PMC article.
-
BST-2/tetherin: a new component of the innate immune response to enveloped viruses.Trends Microbiol. 2010 Sep;18(9):388-96. doi: 10.1016/j.tim.2010.06.010. Epub 2010 Aug 3. Trends Microbiol. 2010. PMID: 20688520 Free PMC article. Review.
-
Single Amino Acid Substitution N659D in HIV-2 Envelope Glycoprotein (Env) Impairs Viral Release and Hampers BST-2 Antagonism.Viruses. 2016 Oct 14;8(10):285. doi: 10.3390/v8100285. Viruses. 2016. PMID: 27754450 Free PMC article.
Cited by
-
Lessons in self-defence: inhibition of virus entry by intrinsic immunity.Nat Rev Immunol. 2022 Jun;22(6):339-352. doi: 10.1038/s41577-021-00626-8. Epub 2021 Oct 13. Nat Rev Immunol. 2022. PMID: 34646033 Free PMC article. Review.
-
HIV-1 Vpr antagonizes innate immune activation by targeting karyopherin-mediated NF-κB/IRF3 nuclear transport.Elife. 2020 Dec 10;9:e60821. doi: 10.7554/eLife.60821. Elife. 2020. PMID: 33300875 Free PMC article.
-
Liquid Biomolecular Condensates and Viral Lifecycles: Review and Perspectives.Viruses. 2021 Feb 25;13(3):366. doi: 10.3390/v13030366. Viruses. 2021. PMID: 33669141 Free PMC article. Review.
-
HIV envelope tail truncation confers resistance to SERINC5 restriction.Proc Natl Acad Sci U S A. 2021 May 25;118(21):e2101450118. doi: 10.1073/pnas.2101450118. Proc Natl Acad Sci U S A. 2021. PMID: 34001619 Free PMC article.
-
CXCR4- and CCR5-Tropic HIV-1 Clones Are Both Tractable to Grow in Rhesus Macaques.Front Microbiol. 2018 Oct 18;9:2510. doi: 10.3389/fmicb.2018.02510. eCollection 2018. Front Microbiol. 2018. PMID: 30405570 Free PMC article.
References
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 6th ed New York: Garland Science; (2014).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

