Cancer treatment in childhood and testicular function: the importance of the somatic environment
- PMID: 29351905
- PMCID: PMC5817964
- DOI: 10.1530/EC-17-0382
Cancer treatment in childhood and testicular function: the importance of the somatic environment
Abstract
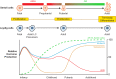
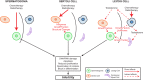
Testicular function and future fertility may be affected by cancer treatment during childhood. Whilst survival of the germ (stem) cells is critical for ensuring the potential for fertility in these patients, the somatic cell populations also play a crucial role in providing a suitable environment to support germ cell maintenance and subsequent development. Regulation of the spermatogonial germ-stem cell niche involves many signalling pathways with hormonal influence from the hypothalamo-pituitary-gonadal axis. In this review, we describe the somatic cell populations that comprise the testicular germ-stem cell niche in humans and how they may be affected by cancer treatment during childhood. We also discuss the experimental models that may be utilized to manipulate the somatic environment and report the results of studies that investigate the potential role of somatic cells in the protection of the germ cells in the testis from cancer treatment.
Keywords: cancer treatment; fertility; fertility preservation; testis.
© 2018 The authors.
Figures
Similar articles
-
Separation of somatic and germ cells is required to establish primate spermatogonial cultures.Hum Reprod. 2014 Sep;29(9):2018-31. doi: 10.1093/humrep/deu157. Epub 2014 Jun 24. Hum Reprod. 2014. PMID: 24963164
-
Fertility restoration with spermatogonial stem cells.Curr Opin Endocrinol Diabetes Obes. 2017 Dec;24(6):424-431. doi: 10.1097/MED.0000000000000370. Curr Opin Endocrinol Diabetes Obes. 2017. PMID: 28938237 Review.
-
Spermatogonial stem cells: updates from specification to clinical relevance.Hum Reprod Update. 2019 May 1;25(3):275-297. doi: 10.1093/humupd/dmz006. Hum Reprod Update. 2019. PMID: 30810745 Review.
-
A diagnostic germ cell score for immature testicular tissue at risk of germ cell loss.Hum Reprod. 2018 Apr 1;33(4):636-645. doi: 10.1093/humrep/dey025. Hum Reprod. 2018. PMID: 29452353
-
Experimental models of testicular development and function using human tissue and cells.Mol Cell Endocrinol. 2018 Jun 15;468:95-110. doi: 10.1016/j.mce.2017.12.011. Epub 2018 Jan 6. Mol Cell Endocrinol. 2018. PMID: 29309804 Review.
Cited by
-
The construction of a testis transcriptional cell atlas from embryo to adult reveals various somatic cells and their molecular roles.J Transl Med. 2023 Nov 27;21(1):859. doi: 10.1186/s12967-023-04722-2. J Transl Med. 2023. PMID: 38012716 Free PMC article.
-
An Assessment of Cryopreserved Semen and Testicular Tissue Collected Before and After Cancer Treatment Initiation.Cancer Manag Res. 2024 Jul 24;16:871-882. doi: 10.2147/CMAR.S460960. eCollection 2024. Cancer Manag Res. 2024. PMID: 39077055 Free PMC article.
-
Spermatogonial quantity in human prepubertal testicular tissue collected for fertility preservation prior to potentially sterilizing therapy.Hum Reprod. 2018 Sep 1;33(9):1677-1683. doi: 10.1093/humrep/dey240. Hum Reprod. 2018. PMID: 30052981 Free PMC article.
-
How does chemotherapy treatment damage the prepubertal testis?Reproduction. 2018 Dec;156(6):R209-R233. doi: 10.1530/REP-18-0221. Reproduction. 2018. PMID: 30394705 Free PMC article. Review.
-
Fertility preservation in boys: recent developments and new insights †.Hum Reprod Open. 2020 Jun 6;2020(3):hoaa016. doi: 10.1093/hropen/hoaa016. eCollection 2020. Hum Reprod Open. 2020. PMID: 32529047 Free PMC article.
References
-
- Anderson RA, Mitchell RT, Kelsey TW, Spears N, Telfer EE, Wallace WH. Cancer treatment and gonadal function: experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes and Endocrinology 2015. 3 556–567. (10.1016/S2213-8587(15)00039-X) - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

