Chanzyme TRPM7 Mediates the Ca2+ Influx Essential for Lipopolysaccharide-Induced Toll-Like Receptor 4 Endocytosis and Macrophage Activation
- PMID: 29343440
- PMCID: PMC5783319
- DOI: 10.1016/j.immuni.2017.11.026
Chanzyme TRPM7 Mediates the Ca2+ Influx Essential for Lipopolysaccharide-Induced Toll-Like Receptor 4 Endocytosis and Macrophage Activation
Abstract
Toll-like receptors (TLRs) sense pathogen-associated molecular patterns to activate the production of inflammatory mediators. TLR4 recognizes lipopolysaccharide (LPS) and drives the secretion of inflammatory cytokines, often contributing to sepsis. We report that transient receptor potential melastatin-like 7 (TRPM7), a non-selective but Ca2+-conducting ion channel, mediates the cytosolic Ca2+ elevations essential for LPS-induced macrophage activation. LPS triggered TRPM7-dependent Ca2+ elevations essential for TLR4 endocytosis and the subsequent activation of the transcription factor IRF3. In a parallel pathway, the Ca2+ signaling initiated by TRPM7 was also essential for the nuclear translocation of NFκB. Consequently, TRPM7-deficient macrophages exhibited major deficits in the LPS-induced transcriptional programs in that they failed to produce IL-1β and other key pro-inflammatory cytokines. In accord with these defects, mice with myeloid-specific deletion of Trpm7 are protected from LPS-induced peritonitis. Our study highlights the importance of Ca2+ signaling in macrophage activation and identifies the ion channel TRPM7 as a central component of TLR4 signaling.
Keywords: LPS; TLR4; TRP channel; TRPM7; endotoxin; inflammation; ion channel; sepsis; toll-like receptor.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
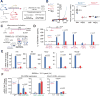

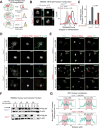
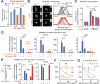
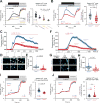

Comment in
-
The Family of LPS Signal Transducers Increases: the Arrival of Chanzymes.Immunity. 2018 Jan 16;48(1):4-6. doi: 10.1016/j.immuni.2017.12.016. Immunity. 2018. PMID: 29343439
Similar articles
-
Endotoxin-induced vascular endothelial cell migration is dependent on TLR4/NF-κB pathway, NAD(P)H oxidase activation, and transient receptor potential melastatin 7 calcium channel activity.Int J Biochem Cell Biol. 2014 Oct;55:11-23. doi: 10.1016/j.biocel.2014.08.001. Epub 2014 Aug 12. Int J Biochem Cell Biol. 2014. PMID: 25130439
-
Phospholipase Cγ-2 and intracellular calcium are required for lipopolysaccharide-induced Toll-like receptor 4 (TLR4) endocytosis and interferon regulatory factor 3 (IRF3) activation.J Biol Chem. 2012 Feb 3;287(6):3704-9. doi: 10.1074/jbc.C111.328559. Epub 2011 Dec 12. J Biol Chem. 2012. PMID: 22158869 Free PMC article.
-
CD14 dependence of TLR4 endocytosis and TRIF signaling displays ligand specificity and is dissociable in endotoxin tolerance.Proc Natl Acad Sci U S A. 2015 Jul 7;112(27):8391-6. doi: 10.1073/pnas.1424980112. Epub 2015 Jun 23. Proc Natl Acad Sci U S A. 2015. PMID: 26106158 Free PMC article.
-
Transient receptor potential melastatin 7 and their modulators.Eur J Pharmacol. 2022 Sep 15;931:175180. doi: 10.1016/j.ejphar.2022.175180. Epub 2022 Aug 5. Eur J Pharmacol. 2022. PMID: 35940237 Review.
-
IRF3 function and immunological gaps in sepsis.Front Immunol. 2024 Feb 5;15:1336813. doi: 10.3389/fimmu.2024.1336813. eCollection 2024. Front Immunol. 2024. PMID: 38375470 Free PMC article. Review.
Cited by
-
TLR4 signalling via Piezo1 engages and enhances the macrophage mediated host response during bacterial infection.Nat Commun. 2021 Jun 10;12(1):3519. doi: 10.1038/s41467-021-23683-y. Nat Commun. 2021. PMID: 34112781 Free PMC article.
-
Negative Regulation of Tec Kinase Alleviates LPS-Induced Acute Kidney Injury in Mice via theTLR4/NF-κB Signaling Pathway.Biomed Res Int. 2020 Jun 19;2020:3152043. doi: 10.1155/2020/3152043. eCollection 2020. Biomed Res Int. 2020. PMID: 32685466 Free PMC article.
-
Endosomal fusion of pH-dependent enveloped viruses requires ion channel TRPM7.Nat Commun. 2024 Oct 1;15(1):8479. doi: 10.1038/s41467-024-52773-w. Nat Commun. 2024. PMID: 39353909 Free PMC article.
-
Beyond the CRAC: Diversification of ion signaling in B cells.Immunol Rev. 2019 Sep;291(1):104-122. doi: 10.1111/imr.12770. Immunol Rev. 2019. PMID: 31402507 Free PMC article. Review.
-
TRP Channels as Sensors of Bacterial Endotoxins.Toxins (Basel). 2018 Aug 11;10(8):326. doi: 10.3390/toxins10080326. Toxins (Basel). 2018. PMID: 30103489 Free PMC article. Review.
References
-
- Afonina IS, Muller C, Martin SJ, Beyaert R. Proteolytic Processing of Interleukin-1 Family Cytokines: Variations on a Common Theme. Immunity. 2015;42:991–1004. - PubMed
-
- Akashi S, Saitoh S, Wakabayashi Y, Kikuchi T, Takamura N, Nagai Y, Kusumoto Y, Fukase K, Kusumoto S, Adachi Y, et al. Lipopolysaccharide Interaction with Cell Surface Toll-like Receptor 4-MD-2: Higher Affinity than That with MD-2 or CD14. The Journal of experimental medicine. 2003;198:1035–1042. - PMC - PubMed
-
- Akashi S, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, Miyake K. Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. Journal of immunology. 2000;164:3471–3475. - PubMed
-
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. The Journal of biological chemistry. 2002;277:21453–21457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

