In Vitro activity of novel glycopolymer against clinical isolates of multidrug-resistant Staphylococcus aureus
- PMID: 29342216
- PMCID: PMC5771624
- DOI: 10.1371/journal.pone.0191522
In Vitro activity of novel glycopolymer against clinical isolates of multidrug-resistant Staphylococcus aureus
Abstract
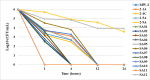
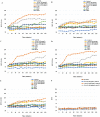
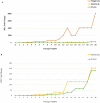
The incidence of multidrug-resistant (MDR) organisms, including methicillin-resistant Staphylococcus aureus (MRSA), is a serious threat to public health. Progress in developing new therapeutics is being outpaced by antibiotic resistance development, and alternative agents that rapidly permeabilize bacteria hold tremendous potential for treating MDR infections. A new class of glycopolymers includes polycationic poly-N (acetyl, arginyl) glucosamine (PAAG) is under development as an alternative to traditional antibiotic strategies to treat MRSA infections. This study demonstrates the antibacterial activity of PAAG against clinical isolates of methicillin and mupirocin-resistant Staphylococcus aureus. Multidrug-resistant S. aureus was rapidly killed by PAAG, which completely eradicated 88% (15/17) of all tested strains (6-log reduction in CFU) in ≤ 12-hours at doses that are non-toxic to mammalian cells. PAAG also sensitized all the clinical MRSA strains (17/17) to oxacillin as demonstrated by the observed reduction in the oxacillin MIC to below the antibiotic resistance breakpoint. The effect of PAAG and standard antibiotics including vancomycin, oxacillin, mupirocin and bacitracin on MRSA permeability was studied by measuring propidium iodide (PI) uptake by bacterial cells. Antimicrobial resistance studies showed that S. aureus developed resistance to PAAG at a rate slower than to mupirocin but similar to bacitracin. PAAG was observed to resensitize drug-resistant S. aureus strains sampled from passage 13 and 20 of the multi-passage resistance study, reducing MICs of mupirocin and bacitracin below their clinical sensitivity breakpoints. This class of bacterial permeabilizing glycopolymers may provide a new tool in the battle against multidrug-resistant bacteria.
Conflict of interest statement
Figures
Similar articles
-
Distribution of high-level mupirocin resistance among clinical MRSA.J Chemother. 2017 Aug;29(4):215-219. doi: 10.1080/1120009X.2016.1201257. Epub 2016 Jul 4. J Chemother. 2017. PMID: 27376552
-
Mupirocin Resistance Among Methicillin Resistant Staphylococcus Isolates in a Tertiary Health Care Center.Infect Disord Drug Targets. 2019;19(2):128-132. doi: 10.2174/1871526518666180501104525. Infect Disord Drug Targets. 2019. PMID: 29714149
-
Association of high-level mupirocin resistance and multidrug-resistant methicillin-resistant Staphylococcus aureus at an academic center in the midwestern United States.J Clin Microbiol. 2011 Jan;49(1):95-100. doi: 10.1128/JCM.00759-10. Epub 2010 Nov 17. J Clin Microbiol. 2011. PMID: 21084520 Free PMC article.
-
Clinical relevance of mupirocin resistance in Staphylococcus aureus.J Hosp Infect. 2013 Dec;85(4):249-56. doi: 10.1016/j.jhin.2013.09.006. Epub 2013 Sep 21. J Hosp Infect. 2013. PMID: 24144552 Review.
-
Antibiotic Resistance in the Treatment of Staphylococcus aureus Keratitis: a 20-Year Review.Cornea. 2015 Jun;34(6):698-703. doi: 10.1097/ICO.0000000000000431. Cornea. 2015. PMID: 25811722 Free PMC article. Review.
Cited by
-
Treatment of Pulmonary Disease of Cystic Fibrosis: A Comprehensive Review.Antibiotics (Basel). 2021 Apr 23;10(5):486. doi: 10.3390/antibiotics10050486. Antibiotics (Basel). 2021. PMID: 33922413 Free PMC article. Review.
-
Improving the antimicrobial efficacy against resistant Staphylococcus aureus by a combined use of conjugated oligoelectrolytes.PLoS One. 2019 Nov 15;14(11):e0224816. doi: 10.1371/journal.pone.0224816. eCollection 2019. PLoS One. 2019. PMID: 31730663 Free PMC article.
-
Polycationic Glycopolymer Demonstrates Activity Against Persisters and Biofilms of Non-tuberculosis Mycobacteria Cystic Fibrosis Clinical Isolates in vitro.Front Microbiol. 2022 Feb 21;13:821820. doi: 10.3389/fmicb.2022.821820. eCollection 2022. Front Microbiol. 2022. PMID: 35265060 Free PMC article.
-
Soluble chitosan derivative treats wound infections and promotes wound healing in a novel MRSA-infected porcine partial-thickness burn wound model.PLoS One. 2022 Oct 14;17(10):e0274455. doi: 10.1371/journal.pone.0274455. eCollection 2022. PLoS One. 2022. PMID: 36240206 Free PMC article.
-
Novel Glycopolymer Eradicates Antibiotic- and CCCP-Induced Persister Cells in Pseudomonas aeruginosa.Front Microbiol. 2018 Aug 3;9:1724. doi: 10.3389/fmicb.2018.01724. eCollection 2018. Front Microbiol. 2018. PMID: 30123191 Free PMC article.
References
-
- David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clinical microbiology reviews. 2010; 1;23(3):616 doi: 10.1128/CMR.00081-09 - DOI - PMC - PubMed
-
- Reddy CM, Thati V, Shivannavar CT, Gaddad SM. Vancomycin resistance among methicillin resistant Staphylococcus aureus isolates in Rayalaseema region Andhra Pradesh, South India. World J Sci Tech. 2012; 2:6–8.
-
- Dasenbrook EC, Checkley W, Merlo CA, Konstan MW, Lechtzin N, Boyle MP. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. Jama. 2010. June 16;303(23):2386–92. doi: 10.1001/jama.2010.791 - DOI - PubMed
-
- Döring G, Flume P, Heijerman H, Elborn JS, Consensus Study Group. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. Journal of Cystic Fibrosis. 2012. December 31;11(6):461–79. doi: 10.1016/j.jcf.2012.10.004 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

