Heteromeric interactions regulate butyrophilin (BTN) and BTN-like molecules governing γδ T cell biology
- PMID: 29339503
- PMCID: PMC5798315
- DOI: 10.1073/pnas.1701237115
Heteromeric interactions regulate butyrophilin (BTN) and BTN-like molecules governing γδ T cell biology
Abstract
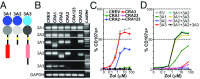
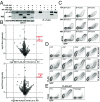
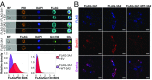
The long-held view that gamma delta (γδ) T cells in mice and humans are fundamentally dissimilar, as are γδ cells in blood and peripheral tissues, has been challenged by emerging evidence of the cells' regulation by butyrophilin (BTN) and butyrophilin-like (BTNL) molecules. Thus, murine Btnl1 and the related gene, Skint1, mediate T cell receptor (TCR)-dependent selection of murine intraepithelial γδ T cell repertoires in gut and skin, respectively; BTNL3 and BTNL8 are TCR-dependent regulators of human gut γδ cells; and BTN3A1 is essential for TCR-dependent activation of human peripheral blood Vγ9Vδ2+ T cells. However, some observations concerning BTN/Btnl molecules continue to question the extent of mechanistic conservation. In particular, murine and human gut γδ cell regulation depends on pairings of Btnl1 and Btnl6 and BTNL3 and BTNL8, respectively, whereas blood γδ cells are reported to be regulated by BTN3A1 independent of other BTNs. Addressing this paradox, we show that BTN3A2 regulates the subcellular localization of BTN3A1, including functionally important associations with the endoplasmic reticulum (ER), and is specifically required for optimal BTN3A1-mediated activation of Vγ9Vδ2+ T cells. Evidence that BTNL3/BTNL8 and Btnl1/Btnl6 likewise associate with the ER reinforces the prospect of broadly conserved mechanisms underpinning the selection and activation of γδ cells in mice and humans, and in blood and extralymphoid sites.
Keywords: butyrophilins; endoplasmic reticulum; evolutionary conservation; gamma delta T cells; zoledronate.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
The Juxtamembrane Domain of Butyrophilin BTN3A1 Controls Phosphoantigen-Mediated Activation of Human Vγ9Vδ2 T Cells.J Immunol. 2017 Jun 1;198(11):4228-4234. doi: 10.4049/jimmunol.1601910. Epub 2017 May 1. J Immunol. 2017. PMID: 28461569
-
Butyrophilin-like 3 Directly Binds a Human Vγ4+ T Cell Receptor Using a Modality Distinct from Clonally-Restricted Antigen.Immunity. 2019 Nov 19;51(5):813-825.e4. doi: 10.1016/j.immuni.2019.09.006. Epub 2019 Oct 15. Immunity. 2019. PMID: 31628053 Free PMC article.
-
Vγ9Vδ2 T cells recognize butyrophilin 2A1 and 3A1 heteromers.Nat Immunol. 2024 Aug;25(8):1355-1366. doi: 10.1038/s41590-024-01892-z. Epub 2024 Jul 16. Nat Immunol. 2024. PMID: 39014161
-
Butyrophilins: γδ T Cell Receptor Ligands, Immunomodulators and More.Front Immunol. 2022 Mar 17;13:876493. doi: 10.3389/fimmu.2022.876493. eCollection 2022. Front Immunol. 2022. PMID: 35371078 Free PMC article. Review.
-
Butyrophilins: Dynamic Regulators of Protective T Cell Immunity in Cancer.Int J Mol Sci. 2023 May 13;24(10):8722. doi: 10.3390/ijms24108722. Int J Mol Sci. 2023. PMID: 37240071 Free PMC article. Review.
Cited by
-
Gamma delta T-cell-based immune checkpoint therapy: attractive candidate for antitumor treatment.Mol Cancer. 2023 Feb 15;22(1):31. doi: 10.1186/s12943-023-01722-0. Mol Cancer. 2023. PMID: 36793048 Free PMC article. Review.
-
Critical Roles for Coiled-Coil Dimers of Butyrophilin 3A1 in the Sensing of Prenyl Pyrophosphates by Human Vγ2Vδ2 T Cells.J Immunol. 2019 Aug 1;203(3):607-626. doi: 10.4049/jimmunol.1801252. Epub 2019 Jun 21. J Immunol. 2019. PMID: 31227581 Free PMC article.
-
Butyrophilin-like 2 regulates site-specific adaptations of intestinal γδ intraepithelial lymphocytes.Commun Biol. 2021 Jul 26;4(1):913. doi: 10.1038/s42003-021-02438-x. Commun Biol. 2021. PMID: 34312491 Free PMC article.
-
γδ T cells in immunotherapies for B-cell malignancies.Front Immunol. 2023 Jun 22;14:1200003. doi: 10.3389/fimmu.2023.1200003. eCollection 2023. Front Immunol. 2023. PMID: 37426670 Free PMC article. Review.
-
NLRC5 promotes transcription of BTN3A1-3 genes and Vγ9Vδ2 T cell-mediated killing.iScience. 2020 Dec 7;24(1):101900. doi: 10.1016/j.isci.2020.101900. eCollection 2021 Jan 22. iScience. 2020. PMID: 33364588 Free PMC article.
References
-
- Hayday AC, et al. Structure, organization, and somatic rearrangement of T cell gamma genes. Cell. 1985;40:259–269. - PubMed
-
- Brenner MB, et al. Identification of a putative second T-cell receptor. Nature. 1986;322:145–149. - PubMed
-
- Hayday A, Vantourout P. A long-playing CD about the γδ TCR repertoire. Immunity. 2013;39:994–996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

