Deregulation of HDAC5 by Viral Interferon Regulatory Factor 3 Plays an Essential Role in Kaposi's Sarcoma-Associated Herpesvirus-Induced Lymphangiogenesis
- PMID: 29339432
- PMCID: PMC5770555
- DOI: 10.1128/mBio.02217-17
Deregulation of HDAC5 by Viral Interferon Regulatory Factor 3 Plays an Essential Role in Kaposi's Sarcoma-Associated Herpesvirus-Induced Lymphangiogenesis
Abstract
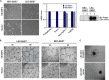
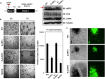
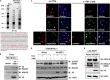
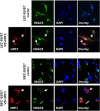
Kaposi's sarcoma-associated herpesvirus (KSHV) is the etiologic agent for Kaposi's sarcoma (KS), which is one of the most common HIV-associated neoplasms. The endothelium is the thin layer of squamous cells where vascular blood endothelial cells (BECs) line the interior surface of blood vessels and lymphatic endothelial cells (LECs) are in direct contact with lymphatic vessels. The KS lesions contain a prominent compartment of neoplastic spindle morphology cells that are closely related to LECs. Furthermore, while KSHV can infect both LECs and BECs in vitro, its infection activates genetic programming related to lymphatic endothelial cell fate, suggesting that lymphangiogenic pathways are involved in KSHV infection and malignancy. Here, we report for the first time that viral interferon regulatory factor 3 (vIRF3) is readily detected in over 40% of KS lesions and that vIRF3 functions as a proangiogenic factor, inducing hypersprouting formation and abnormal growth in a LEC-specific manner. Mass spectrometry analysis revealed that vIRF3 interacted with histone deacetylase 5 (HDAC5), which is a signal-responsive regulator for vascular homeostasis. This interaction blocked the phosphorylation-dependent cytosolic translocation of HDAC5 and ultimately altered global gene expression in LECs but not in BECs. Consequently, vIRF3 robustly induced spindle morphology and hypersprouting formation of LECs but not BECs. Finally, KSHV infection led to the hypersprouting formation of LECs, whereas infection with a ΔvIRF3 mutant did not do so. Collectively, our data indicate that vIRF3 alters global gene expression and induces a hypersprouting formation in an HDAC5-binding-dependent and LEC-specific manner, ultimately contributing to KSHV-associated pathogenesis.IMPORTANCE Several lines of evidences indicate that KSHV infection of LECs induces pathological lymphangiogenesis and that the results resemble KS-like spindle morphology. However, the underlying molecular mechanism remains unclear. Here, we demonstrated that KSHV vIRF3 is readily detected in over 40% of various KS lesions and functions as a potent prolymphangiogenic factor by blocking the phosphorylation-dependent cytosolic translocation of HDAC5, which in turn modulates global gene expression in LECs. Consequently, vIRF3-HDAC5 interaction contributes to virus-induced lymphangiogenesis. The results of this study suggest that KSHV vIRF3 plays a crucial role in KSHV-induced malignancy.
Keywords: Kaposi's sarcoma-associated herpesvirus; angiogenesis; histone deacetylase.
Copyright © 2018 Lee et al.
Figures


Similar articles
-
Kaposi's Sarcoma-Associated Herpesvirus Infection Induces the Expression of Neuroendocrine Genes in Endothelial Cells.J Virol. 2020 Mar 31;94(8):e01692-19. doi: 10.1128/JVI.01692-19. Print 2020 Mar 31. J Virol. 2020. PMID: 31969437 Free PMC article.
-
Ets-1 is required for the activation of VEGFR3 during latent Kaposi's sarcoma-associated herpesvirus infection of endothelial cells.J Virol. 2013 Jun;87(12):6758-68. doi: 10.1128/JVI.03241-12. Epub 2013 Apr 3. J Virol. 2013. PMID: 23552426 Free PMC article.
-
vIRF3 encoded by Kaposi's sarcoma-associated herpesvirus inhibits T-cell factor-dependent transcription via a CREB-binding protein-interaction motif.Biochem Biophys Res Commun. 2016 Oct 28;479(4):697-702. doi: 10.1016/j.bbrc.2016.09.150. Epub 2016 Sep 28. Biochem Biophys Res Commun. 2016. PMID: 27693583
-
Cellular origin of Kaposi's sarcoma and Kaposi's sarcoma-associated herpesvirus-induced cell reprogramming.Trends Cell Biol. 2013 Sep;23(9):421-32. doi: 10.1016/j.tcb.2013.04.001. Epub 2013 May 17. Trends Cell Biol. 2013. PMID: 23685018 Review.
-
Kaposi's sarcoma herpesvirus-induced endothelial cell reprogramming supports viral persistence and contributes to Kaposi's sarcoma tumorigenesis.Curr Opin Virol. 2017 Oct;26:156-162. doi: 10.1016/j.coviro.2017.09.002. Epub 2017 Oct 12. Curr Opin Virol. 2017. PMID: 29031103 Review.
Cited by
-
Sirtuin 6 Attenuates Kaposi's Sarcoma-Associated Herpesvirus Reactivation by Suppressing Ori-Lyt Activity and Expression of RTA.J Virol. 2019 Mar 21;93(7):e02200-18. doi: 10.1128/JVI.02200-18. Print 2019 Apr 1. J Virol. 2019. PMID: 30651359 Free PMC article.
-
NDRG1 facilitates the replication and persistence of Kaposi's sarcoma-associated herpesvirus by interacting with the DNA polymerase clamp PCNA.PLoS Pathog. 2019 Feb 27;15(2):e1007628. doi: 10.1371/journal.ppat.1007628. eCollection 2019 Feb. PLoS Pathog. 2019. PMID: 30811506 Free PMC article.
-
Comparative analysis of the viral interferon regulatory factors of KSHV for their requisite for virus production and inhibition of the type I interferon pathway.Virology. 2020 Feb;541:160-173. doi: 10.1016/j.virol.2019.12.011. Epub 2019 Dec 30. Virology. 2020. PMID: 32056714 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen dysregulates expression of MCL-1 by targeting FBW7.PLoS Pathog. 2021 Jan 20;17(1):e1009179. doi: 10.1371/journal.ppat.1009179. eCollection 2021 Jan. PLoS Pathog. 2021. PMID: 33471866 Free PMC article.
-
Viral Manipulation of the Host Epigenome as a Driver of Virus-Induced Oncogenesis.Microorganisms. 2021 May 30;9(6):1179. doi: 10.3390/microorganisms9061179. Microorganisms. 2021. PMID: 34070716 Free PMC article. Review.
References
-
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d’Agay MF, Clauvel JP, Raphael M, Degos L, Sigaux F. 1995. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood 86:1276–1280. - PubMed
-
- McAllister SC, Moses AV. 2007. Endothelial cell- and lymphocyte-based in vitro systems for understanding KSHV biology. Curr Top Microbiol Immunol 312:211–244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA197153/CA/NCI NIH HHS/United States
- R01 CA096512/CA/NCI NIH HHS/United States
- R21 AI129496/AI/NIAID NIH HHS/United States
- R21 DE027888/DE/NIDCR NIH HHS/United States
- R01 DE025465/DE/NIDCR NIH HHS/United States
- R01 AI073099/AI/NIAID NIH HHS/United States
- R01 CA132637/CA/NCI NIH HHS/United States
- R35 CA200422/CA/NCI NIH HHS/United States
- UM1 AI106716/AI/NIAID NIH HHS/United States
- R01 AI116585/AI/NIAID NIH HHS/United States
- R01 CA213275/CA/NCI NIH HHS/United States
- R01 DE023926/DE/NIDCR NIH HHS/United States
- P01 CA180779/CA/NCI NIH HHS/United States
- R21 EY026260/EY/NEI NIH HHS/United States
- R01 HL121036/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

