Nephronectin Regulates Mesangial Cell Adhesion and Behavior in Glomeruli
- PMID: 29335243
- PMCID: PMC5875951
- DOI: 10.1681/ASN.2017070752
Nephronectin Regulates Mesangial Cell Adhesion and Behavior in Glomeruli
Abstract
A critical aspect of kidney function occurs at the glomerulus, the capillary network that filters the blood. The glomerular basement membrane (GBM) is a key component of filtration, yet our understanding of GBM interactions with mesangial cells, specialized pericytes that provide structural stability to glomeruli, is limited. We investigated the role of nephronectin (Npnt), a GBM component and known ligand of α8β1 integrin. Immunolocalization and in situ hybridization studies in kidneys of adult mice revealed that nephronectin is produced by podocytes and deposited into the GBM. Conditional deletion of Npnt from nephron progenitors caused a pronounced increase in mesangial cell number and mesangial sclerosis. Nephronectin colocalized with α8β1 integrin to novel, specialized adhesion structures that occurred at sites of mesangial cell protrusion at the base of the capillary loops. Absence of nephronectin disrupted these adhesion structures, leading to mislocalization of α8β1. Podocyte-specific deletion of Npnt also led to mesangial sclerosis in mice. These results demonstrate a novel role for nephronectin and α8β1 integrin in a newly described adhesion complex and begin to uncover the molecular interactions between the GBM and mesangial cells, which govern mesangial cell behavior and may have a role in pathologic states.
Keywords: cell biology and structure; cell-matrix-interactions; glomerular basement membrane; mesangial cells; nephronectin.
Copyright © 2018 by the American Society of Nephrology.
Figures

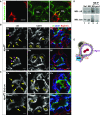
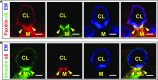

Comment in
-
Maintenance and Breakdown of Glomerular Tuft Architecture.J Am Soc Nephrol. 2018 Apr;29(4):1075-1077. doi: 10.1681/ASN.2018020200. Epub 2018 Mar 13. J Am Soc Nephrol. 2018. PMID: 29535166 Free PMC article. No abstract available.
Similar articles
-
Glomerular Endothelial Cell-Derived microRNA-192 Regulates Nephronectin Expression in Idiopathic Membranous Glomerulonephritis.J Am Soc Nephrol. 2021 Nov;32(11):2777-2794. doi: 10.1681/ASN.2020121699. J Am Soc Nephrol. 2021. PMID: 34716242 Free PMC article.
-
Mesangial cells organize the glomerular capillaries by adhering to the G domain of laminin alpha5 in the glomerular basement membrane.J Cell Biol. 2003 Apr 14;161(1):187-96. doi: 10.1083/jcb.200211121. Epub 2003 Apr 7. J Cell Biol. 2003. PMID: 12682087 Free PMC article.
-
Abnormal glomerular basement membrane maturation impairs mesangial cell differentiation during murine postnatal nephrogenesis.Am J Physiol Renal Physiol. 2023 Jan 1;324(1):F124-F134. doi: 10.1152/ajprenal.00192.2022. Epub 2022 Nov 23. Am J Physiol Renal Physiol. 2023. PMID: 36417276
-
Differential role of mesangial cells and podocytes in TGF-beta-induced mesangial matrix synthesis in chronic glomerular disease.Histol Histopathol. 2009 Jul;24(7):901-8. doi: 10.14670/HH-24.901. Histol Histopathol. 2009. PMID: 19475536 Review.
-
Mesangial cells and their adhesive properties.Exp Nephrol. 1999 Mar-Apr;7(2):137-46. doi: 10.1159/000020594. Exp Nephrol. 1999. PMID: 10213867 Review.
Cited by
-
Identification of an Altered Matrix Signature in Kidney Aging and Disease.J Am Soc Nephrol. 2021 Jul;32(7):1713-1732. doi: 10.1681/ASN.2020101442. Epub 2021 May 28. J Am Soc Nephrol. 2021. PMID: 34049963 Free PMC article.
-
A cell-ECM mechanism for connecting the ipsilateral eye to the brain.Proc Natl Acad Sci U S A. 2021 Oct 19;118(42):e2104343118. doi: 10.1073/pnas.2104343118. Proc Natl Acad Sci U S A. 2021. PMID: 34654745 Free PMC article.
-
Maintenance and Breakdown of Glomerular Tuft Architecture.J Am Soc Nephrol. 2018 Apr;29(4):1075-1077. doi: 10.1681/ASN.2018020200. Epub 2018 Mar 13. J Am Soc Nephrol. 2018. PMID: 29535166 Free PMC article. No abstract available.
-
Nephronectin-integrin α8 signaling is required for proper migration of periocular neural crest cells during chick corneal development.Elife. 2022 Mar 3;11:e74307. doi: 10.7554/eLife.74307. Elife. 2022. PMID: 35238772 Free PMC article.
-
Spatially Resolved Transcriptomes of Mammalian Kidneys Illustrate the Molecular Complexity and Interactions of Functional Nephron Segments.Front Med (Lausanne). 2022 Jul 7;9:873923. doi: 10.3389/fmed.2022.873923. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35872784 Free PMC article.
References
-
- Schlöndorff D, Banas B: The mesangial cell revisited: No cell is an island. J Am Soc Nephrol 20: 1179–1187, 2009 - PubMed
-
- Miner JH: Developmental biology of glomerular basement membrane components. Curr Opin Nephrol Hypertens 7: 13–19, 1998 - PubMed
-
- Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, et al. : Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science 248: 1224–1227, 1990 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

