Regulation of vascular tone homeostasis by NO and H2S: Implications in hypertension
- PMID: 29330066
- PMCID: PMC5866223
- DOI: 10.1016/j.bcp.2018.01.017
Regulation of vascular tone homeostasis by NO and H2S: Implications in hypertension
Abstract
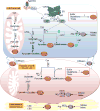
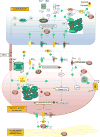
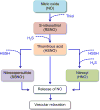
Nitric oxide (NO) and hydrogen sulfide (H2S) are two gasotransmitters that are produced in the vasculature and contribute to the regulation of vascular tone. NO and H2S are synthesized in both vascular smooth muscle and endothelial cells; NO functions primarily through the sGC/cGMP pathway, and H2S mainly through activation of the ATP-dependent potassium channels; both leading to relaxation of vascular smooth muscle cells. A deficit in the NO/H2S homeostasis is involved in the pathogenesis of various cardiovascular diseases, especially hypertension. It is now becoming increasingly clear that there are important interactions between NO and H2S and that have a profound impact on vascular tone and this may provide insights into the new therapeutic interventions. The aim of this review is to provide a better understanding of individual and interactive roles of NO and H2S in vascular biology. Overall, available data indicate that both NO and H2S contribute to vascular (patho)physiology and in regulating blood pressure. In addition, boosting NO and H2S using various dietary sources or donors could be a hopeful therapeutic strategy in the management of hypertension.
Keywords: Cell signaling; Hydrogen sulfide; Hypertension; Nitric oxide; Protein modification; Vascular tone.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
None
Figures

Similar articles
-
Interaction of Hydrogen Sulfide with Nitric Oxide in the Cardiovascular System.Oxid Med Cell Longev. 2016;2016:6904327. doi: 10.1155/2016/6904327. Epub 2015 Nov 10. Oxid Med Cell Longev. 2016. PMID: 26640616 Free PMC article. Review.
-
An Update on Hydrogen Sulfide and Nitric Oxide Interactions in the Cardiovascular System.Oxid Med Cell Longev. 2018 Sep 9;2018:4579140. doi: 10.1155/2018/4579140. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30271527 Free PMC article. Review.
-
[Molecular Mechanisms of Action of Gas Transmitters NO, CO and H2S in Smooth Muscle Cells and Effect of NO-generating Compounds (Nitrates and Nitrites) on Average Life Expectancy].Usp Fiziol Nauk. 2017 Jan-Mar;48(1):24-52. Usp Fiziol Nauk. 2017. PMID: 29283238 Review. Russian.
-
Cystathionine γ-Lyase-Produced Hydrogen Sulfide Controls Endothelial NO Bioavailability and Blood Pressure.Hypertension. 2018 Jun;71(6):1210-1217. doi: 10.1161/HYPERTENSIONAHA.117.10562. Epub 2018 Apr 30. Hypertension. 2018. PMID: 29712741
-
Regulation of carbohydrate metabolism by nitric oxide and hydrogen sulfide: Implications in diabetes.Biochem Pharmacol. 2020 Jun;176:113819. doi: 10.1016/j.bcp.2020.113819. Epub 2020 Jan 21. Biochem Pharmacol. 2020. PMID: 31972170 Free PMC article. Review.
Cited by
-
Ecklonia cava Extract and Its Derivative Dieckol Promote Vasodilation by Modulating Calcium Signaling and PI3K/AKT/eNOS Pathway in In Vitro and In Vivo Models.Biomedicines. 2021 Apr 19;9(4):438. doi: 10.3390/biomedicines9040438. Biomedicines. 2021. PMID: 33921856 Free PMC article.
-
Role of hydrogen sulfide in sulfur dioxide production and vascular regulation.PLoS One. 2022 Mar 17;17(3):e0264891. doi: 10.1371/journal.pone.0264891. eCollection 2022. PLoS One. 2022. PMID: 35298485 Free PMC article.
-
Elucidating VSMC phenotypic transition mechanisms to bridge insights into cardiovascular disease implications.Front Cardiovasc Med. 2024 May 13;11:1400780. doi: 10.3389/fcvm.2024.1400780. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38803664 Free PMC article. Review.
-
Metabolomics of repetitive myocardial stunning in chronic multivessel coronary artery stenosis: Effect of non-selective and selective β1-receptor blockers.J Physiol. 2024 Jul;602(14):3423-3448. doi: 10.1113/JP285720. Epub 2024 Jun 17. J Physiol. 2024. PMID: 38885335
-
GYY4137 and Sodium Hydrogen Sulfide Relaxations Are Inhibited by L-Cysteine and KV7 Channel Blockers in Rat Small Mesenteric Arteries.Front Pharmacol. 2021 Mar 26;12:613989. doi: 10.3389/fphar.2021.613989. eCollection 2021. Front Pharmacol. 2021. PMID: 33841145 Free PMC article.
References
-
- Moccia F, Bertoni G, Pla AF, Dragoni S, Pupo E, Merlino A, et al. Hydrogen sulfide regulates intracellular Ca2+ concentration in endothelial cells from excised rat aorta. Current pharmaceutical biotechnology. 2011;12:1416–26. - PubMed
-
- Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino Acids. 2004;26:243–54. - PubMed
-
- Martelli A, Testai L, Breschi MC, Lawson K, McKay NG, Miceli F, et al. Vasorelaxation by hydrogen sulphide involves activation of Kv7 potassium channels. Pharmacological research. 2013;70:27–34. - PubMed
-
- Kubo S, Kurokawa Y, Doe I, Masuko T, Sekiguchi F, Kawabata A. Hydrogen sulfide inhibits activity of three isoforms of recombinant nitric oxide synthase. Toxicology. 2007;241:92–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

