The Endosomal Protein Endotubin Is Required for Enterocyte Differentiation
- PMID: 29322087
- PMCID: PMC5756061
- DOI: 10.1016/j.jcmgh.2017.11.001
The Endosomal Protein Endotubin Is Required for Enterocyte Differentiation
Abstract
Background & aims: During late embryonic development and through weaning, enterocytes of the ileum are highly endocytic. Defects in endocytosis and trafficking are implicated in neonatal disease, however, the mechanisms regulating trafficking during the developmental period are incompletely understood. The apical endosomal protein endotubin (EDTB) is highly expressed in the late embryonic and neonatal ileum. In epithelial cells in vitro, EDTB regulates both trafficking of tight junction proteins and proliferation through modulation of YAP activity. However, EDTB function during the endocytic stage of development of the intestine is unknown.
Methods: By using Villin-CreERT2, we induced knockout of EDTB during late gestation and analyzed the impact on endocytic compartments and enterocyte structure in neonates using immunofluorescence, immunocytochemistry, and transmission electron microscopy.
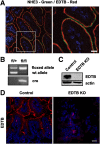
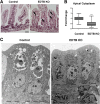
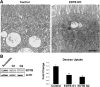
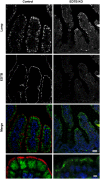
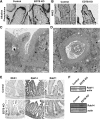
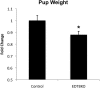
Results: Deletion of the apical endosomal protein EDTB in the small intestine during development impairs enterocyte morphogenesis, including loss of the apical endocytic complex, defective formation of the lysosomal compartment, and some cells had large microvillus-rich inclusions similar to those observed in microvillus inclusion disease. There also was a decrease in apical endocytosis and mislocalization of proteins involved in apical trafficking.
Conclusions: Our results show that EDTB-mediated trafficking within the epithelial cells of the developing ileum is important for maintenance of endocytic compartments and enterocyte integrity during early stages of gut development.
Keywords: AEC, apical endocytic complex; AP, alkaline phosphatase; CRISPR/Cas9, clustered regularly interspaced short palindromic repeats/cas9 endonuclease; EDTB, endotubin; EEA1, early endosomal antigen 1; Endosomes; Endotubin; G, guide; GFP, green fluorescent protein; GTPase, guanosine triphosphatase; KO, knockout; LAMP1, lysosome-associated membrane protein 1; MAMDC4, MAM domain containing 4; MVID, microvillus inclusion disease; P, postnatal day; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; Rab; SDS, sodium dodecyl sulfate; TBST, tris-buffered saline with 0.1% tween-20; TEM, transmission electron microscopic; TJ, tight junction; Tight Junction; Trafficking.
Figures

Similar articles
-
Proliferation in the developing intestine is regulated by the endosomal protein Endotubin.Dev Biol. 2021 Dec;480:50-61. doi: 10.1016/j.ydbio.2021.08.009. Epub 2021 Aug 17. Dev Biol. 2021. PMID: 34411593 Free PMC article.
-
Dynamic Formation of Microvillus Inclusions During Enterocyte Differentiation in Munc18-2-Deficient Intestinal Organoids.Cell Mol Gastroenterol Hepatol. 2018 Aug 14;6(4):477-493.e1. doi: 10.1016/j.jcmgh.2018.08.001. eCollection 2018. Cell Mol Gastroenterol Hepatol. 2018. PMID: 30364784 Free PMC article.
-
Recruitment of Polarity Complexes and Tight Junction Proteins to the Site of Apical Bulk Endocytosis.Cell Mol Gastroenterol Hepatol. 2021;12(1):59-80. doi: 10.1016/j.jcmgh.2021.01.022. Epub 2021 Feb 3. Cell Mol Gastroenterol Hepatol. 2021. PMID: 33548596 Free PMC article.
-
Altered MYO5B Function Underlies Microvillus Inclusion Disease: Opportunities for Intervention at a Cellular Level.Cell Mol Gastroenterol Hepatol. 2022;14(3):553-565. doi: 10.1016/j.jcmgh.2022.04.015. Epub 2022 Jun 1. Cell Mol Gastroenterol Hepatol. 2022. PMID: 35660026 Free PMC article. Review.
-
Endocytic pathways and endosomal trafficking: a primer.Wien Med Wochenschr. 2016 May;166(7-8):196-204. doi: 10.1007/s10354-016-0432-7. Epub 2016 Feb 9. Wien Med Wochenschr. 2016. PMID: 26861668 Free PMC article. Review.
Cited by
-
Maf family transcription factors are required for nutrient uptake in the mouse neonatal gut.Development. 2022 Dec 1;149(23):dev201251. doi: 10.1242/dev.201251. Epub 2022 Dec 12. Development. 2022. PMID: 36504079 Free PMC article.
-
Formation of Giant Lysosome in Neonatal Ileal Enterocytes Requires Endotubin.Cell Mol Gastroenterol Hepatol. 2017 Nov 27;5(2):167-168. doi: 10.1016/j.jcmgh.2017.11.002. eCollection 2018. Cell Mol Gastroenterol Hepatol. 2017. PMID: 29693046 Free PMC article. No abstract available.
-
Loss of MYO5B Leads to Reductions in Na+ Absorption With Maintenance of CFTR-Dependent Cl- Secretion in Enterocytes.Gastroenterology. 2018 Dec;155(6):1883-1897.e10. doi: 10.1053/j.gastro.2018.08.025. Epub 2018 Aug 23. Gastroenterology. 2018. PMID: 30144427 Free PMC article.
-
Proliferation in the developing intestine is regulated by the endosomal protein Endotubin.Dev Biol. 2021 Dec;480:50-61. doi: 10.1016/j.ydbio.2021.08.009. Epub 2021 Aug 17. Dev Biol. 2021. PMID: 34411593 Free PMC article.
-
Modeling the cell biology of monogenetic intestinal epithelial disorders.J Cell Biol. 2024 Jul 1;223(7):e202310118. doi: 10.1083/jcb.202310118. Epub 2024 Apr 29. J Cell Biol. 2024. PMID: 38683247 Free PMC article. Review.
References
-
- Erickson R.P., Larson-Thome K., Valenzuela R.K., Whitaker S.E., Shub M.D. Navajo microvillous inclusion disease is due to a mutation in MYO5B. Am J Med Genet A. 2008;146A:3117–3119. - PubMed
-
- Hackam D.J., Upperman J.S., Grishin A., Ford H.R. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:49–57. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

