Membrane shape-mediated wave propagation of cortical protein dynamics
- PMID: 29321558
- PMCID: PMC5762918
- DOI: 10.1038/s41467-017-02469-1
Membrane shape-mediated wave propagation of cortical protein dynamics
Abstract
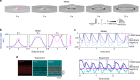
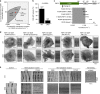
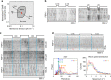
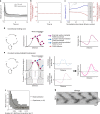
Immune cells exhibit stimulation-dependent traveling waves in the cortex, much faster than typical cortical actin waves. These waves reflect rhythmic assembly of both actin machinery and peripheral membrane proteins such as F-BAR domain-containing proteins. Combining theory and experiments, we develop a mechanochemical feedback model involving membrane shape changes and F-BAR proteins that render the cortex an interesting dynamical system. We show that such cortical dynamics manifests itself as ultrafast traveling waves of cortical proteins, in which the curvature sensitivity-driven feedback always constrains protein lateral diffusion in wave propagation. The resulting protein wave propagation mainly reflects the spatial gradient in the timing of local protein recruitment from cytoplasm. We provide evidence that membrane undulations accompany these protein waves and potentiate their propagation. Therefore, membrane shape change and protein curvature sensitivity may have underappreciated roles in setting high-speed cortical signal transduction rhythms.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Comment in
-
The Rise of Ultrafast Waves.Dev Cell. 2018 Dec 3;47(5):532-534. doi: 10.1016/j.devcel.2018.11.026. Dev Cell. 2018. PMID: 30513294 Free PMC article.
Similar articles
-
Plakoglobin is required for maintenance of the cortical actin skeleton in early Xenopus embryos and for cdc42-mediated wound healing.J Cell Biol. 2002 Aug 19;158(4):695-708. doi: 10.1083/jcb.200202123. Epub 2002 Aug 19. J Cell Biol. 2002. PMID: 12186853 Free PMC article.
-
An actin-based wave generator organizes cell motility.PLoS Biol. 2007 Sep;5(9):e221. doi: 10.1371/journal.pbio.0050221. PLoS Biol. 2007. PMID: 17696648 Free PMC article.
-
Reaction-diffusion waves coupled with membrane curvature.Soft Matter. 2021 Jul 14;17(27):6589-6596. doi: 10.1039/d1sm00540e. Soft Matter. 2021. PMID: 34166481
-
When Physics Takes Over: BAR Proteins and Membrane Curvature.Trends Cell Biol. 2015 Dec;25(12):780-792. doi: 10.1016/j.tcb.2015.09.005. Epub 2015 Oct 28. Trends Cell Biol. 2015. PMID: 26519988 Free PMC article. Review.
-
Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides.Physiol Rev. 2010 Jan;90(1):259-89. doi: 10.1152/physrev.00036.2009. Physiol Rev. 2010. PMID: 20086078 Review.
Cited by
-
Curvature increases permeability of the plasma membrane for ions, water and the anti-cancer drugs cisplatin and gemcitabine.Sci Rep. 2019 Nov 20;9(1):17214. doi: 10.1038/s41598-019-53952-2. Sci Rep. 2019. PMID: 31748538 Free PMC article.
-
Moving through a changing world: Single cell migration in 2D vs. 3D.Front Cell Dev Biol. 2022 Dec 20;10:1080995. doi: 10.3389/fcell.2022.1080995. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36605722 Free PMC article. Review.
-
Microtopographical guidance of macropinocytic signaling patches.Proc Natl Acad Sci U S A. 2021 Dec 14;118(50):e2110281118. doi: 10.1073/pnas.2110281118. Proc Natl Acad Sci U S A. 2021. PMID: 34876521 Free PMC article.
-
Protein-membrane interactions: sensing and generating curvature.Trends Biochem Sci. 2024 May;49(5):401-416. doi: 10.1016/j.tibs.2024.02.005. Epub 2024 Mar 19. Trends Biochem Sci. 2024. PMID: 38508884 Review.
-
Research progress on the regulatory role of cell membrane surface tension in cell behavior.Heliyon. 2024 Apr 26;10(9):e29923. doi: 10.1016/j.heliyon.2024.e29923. eCollection 2024 May 15. Heliyon. 2024. PMID: 38720730 Free PMC article. Review.
References
-
- Winfree A. T. The Geometry of Biological Time, 2nd edn. Springer (2001).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

