NF-kappaB: Two Sides of the Same Coin
- PMID: 29315242
- PMCID: PMC5793177
- DOI: 10.3390/genes9010024
NF-kappaB: Two Sides of the Same Coin
Abstract
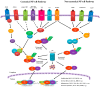
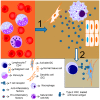
Nuclear Factor-kappa B (NF-κB) is a transcription factor family that regulates a large number of genes that are involved in important physiological processes, including survival, inflammation, and immune responses. More recently, constitutive expression of NF-κB has been associated with several types of cancer. In addition, microorganisms, such as viruses and bacteria, cooperate in the activation of NF-κB in tumors, confirming the multifactorial role of this transcription factor as a cancer driver. Recent reports have shown that the NF-κB signaling pathway should receive attention for the development of therapies. In addition to the direct effects of NF-κB in cancer cells, it might also impact immune cells that can both promote or prevent tumor development. Currently, with the rise of cancer immunotherapy, the link among immune cells, inflammation, and cancer is a major focus, and NF-κB could be an important regulator for the success of these therapies. This review discusses the contrasting roles of NF-κB as a regulator of pro- and antitumor processes and its potential as a therapeutic target.
Keywords: NF-kB; NF-kappaB; cancer; immune response; inflammation; signaling; therapy; treatment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Evidence for the Involvement of the Master Transcription Factor NF-κB in Cancer Initiation and Progression.Biomedicines. 2018 Jul 27;6(3):82. doi: 10.3390/biomedicines6030082. Biomedicines. 2018. PMID: 30060453 Free PMC article. Review.
-
The Role of BMP Signaling and NF-κB Signaling on Osteoblastic Differentiation, Cancer Development, and Vascular Diseases--Is the Activation of NF-κB a Friend or Foe of BMP Function?Vitam Horm. 2015;99:145-70. doi: 10.1016/bs.vh.2015.05.002. Epub 2015 Jun 26. Vitam Horm. 2015. PMID: 26279375 Review.
-
Invasion associated up-regulation of nuclear factor kappaB target genes in colorectal cancer.Cancer. 2009 Nov 1;115(21):4946-58. doi: 10.1002/cncr.24564. Cancer. 2009. PMID: 19658179
-
Nuclear factor-kappaB in development, prevention, and therapy of cancer.Clin Cancer Res. 2007 Feb 15;13(4):1076-82. doi: 10.1158/1078-0432.CCR-06-2221. Clin Cancer Res. 2007. PMID: 17317814 Review.
-
Analysis of the TLR/NF-kappaB pathway in antigen-presenting cells in malignancies promoted by inflammation.Methods Mol Biol. 2009;512:99-117. doi: 10.1007/978-1-60327-530-9_7. Methods Mol Biol. 2009. PMID: 19347275
Cited by
-
Tanshinone IIA Protects Against Cerebral Ischemia Reperfusion Injury by Regulating Microglial Activation and Polarization via NF-κB Pathway.Front Pharmacol. 2021 Apr 19;12:641848. doi: 10.3389/fphar.2021.641848. eCollection 2021. Front Pharmacol. 2021. PMID: 33953677 Free PMC article.
-
NF-kappa B Signaling-Related Signatures Are Connected with the Mesenchymal Phenotype of Circulating Tumor Cells in Non-Metastatic Breast Cancer.Cancers (Basel). 2019 Dec 6;11(12):1961. doi: 10.3390/cancers11121961. Cancers (Basel). 2019. PMID: 31817685 Free PMC article.
-
Mechanisms of Immunomodulation and Cytoprotection Conferred to Pancreatic Islet by Human Amniotic Epithelial Cells.Stem Cell Rev Rep. 2022 Jan;18(1):346-359. doi: 10.1007/s12015-021-10269-w. Epub 2021 Oct 6. Stem Cell Rev Rep. 2022. PMID: 34613550 Free PMC article.
-
A hypothesis for the pathogenesis of radiation-induced oral mucositis: when biological challenges exceed physiologic protective mechanisms. Implications for pharmacological prevention and treatment.Support Care Cancer. 2021 Sep;29(9):4939-4947. doi: 10.1007/s00520-021-06108-w. Epub 2021 Mar 13. Support Care Cancer. 2021. PMID: 33712912 Free PMC article. Review.
-
Targeting NF-κB Signaling for Multiple Myeloma.Cancers (Basel). 2020 Aug 6;12(8):2203. doi: 10.3390/cancers12082203. Cancers (Basel). 2020. PMID: 32781681 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

