H3 relaxin inhibits the collagen synthesis via ROS- and P2X7R-mediated NLRP3 inflammasome activation in cardiac fibroblasts under high glucose
- PMID: 29314607
- PMCID: PMC5824385
- DOI: 10.1111/jcmm.13464
H3 relaxin inhibits the collagen synthesis via ROS- and P2X7R-mediated NLRP3 inflammasome activation in cardiac fibroblasts under high glucose
Erratum in
-
Corrigendum: H3 relaxin inhibits the collagen synthesis via ROS- and P2X7R-mediated NLRP3 inflammasome activation in cardiac fibroblasts under high glucose.J Cell Mol Med. 2018 Jun;22(6):3264-3265. doi: 10.1111/jcmm.13615. J Cell Mol Med. 2018. PMID: 29878720 Free PMC article. No abstract available.
Abstract
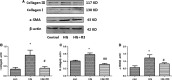
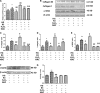
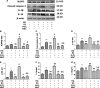
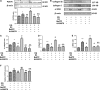
Excessive production of reactive oxygen species (ROS) and P2X7R activation induced by high glucose increases NLRP3 inflammasome activation, which contributes to the pathogenesis of diabetic cardiomyopathy. Although H3 relaxin has been shown to inhibit cardiac fibrosis induced by isoproterenol, the mechanism has not been well studied. Here, we demonstrated that high glucose (HG) induced the collagen synthesis by activation of the NLRP3 inflammasome, leading to caspase-1 activation, interleukin-1β (IL-1β) and IL-18 secretion in neonatal rat cardiac fibroblasts. Moreover, we used a high-glucose model with neonatal rat cardiac fibroblasts and showed that the activation of ROS and P2X7R was augmented and that ROS- and P2X7R-mediated NLRP3 inflammasome activation was critical for the collagen synthesis. Inhibition of ROS and P2X7R decreased NLRP3 inflammasome-mediated collagen synthesis, similar to the effects of H3 relaxin. Furthermore, H3 relaxin reduced the collagen synthesis via ROS- and P2X7R-mediated NLRP3 inflammasome activation in response to HG. These results provide a mechanism by which H3 relaxin alleviates NLRP3 inflammasome-mediated collagen synthesis through the inhibition of ROS and P2X7R under HG conditions and suggest that H3 relaxin represents a potential drug for alleviating cardiac fibrosis in diabetic cardiomyopathy.
Keywords: NLRP3 inflammasome; P2X7R; ROS; cardiac fibrosis; high glucose.
© 2018 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

Similar articles
-
Gypenosides improve diabetic cardiomyopathy by inhibiting ROS-mediated NLRP3 inflammasome activation.J Cell Mol Med. 2018 Sep;22(9):4437-4448. doi: 10.1111/jcmm.13743. Epub 2018 Jul 11. J Cell Mol Med. 2018. PMID: 29993180 Free PMC article.
-
Relaxin Inhibits the Cardiac Myofibroblast NLRP3 Inflammasome as Part of Its Anti-Fibrotic Actions via the Angiotensin Type 2 and ATP (P2X7) Receptors.Int J Mol Sci. 2022 Jun 25;23(13):7074. doi: 10.3390/ijms23137074. Int J Mol Sci. 2022. PMID: 35806076 Free PMC article.
-
Potentiation of hepatic stellate cell activation by extracellular ATP is dependent on P2X7R-mediated NLRP3 inflammasome activation.Pharmacol Res. 2017 Mar;117:82-93. doi: 10.1016/j.phrs.2016.11.040. Epub 2016 Dec 8. Pharmacol Res. 2017. PMID: 27940204
-
The Role of NLRP3 Inflammasome in Cerebrovascular Diseases Pathology and Possible Therapeutic Targets.ASN Neuro. 2021 Jan-Dec;13:17590914211018100. doi: 10.1177/17590914211018100. ASN Neuro. 2021. PMID: 34053242 Free PMC article. Review.
-
NLRP3 Inflammasome in Diabetic Cardiomyopathy and Exercise Intervention.Int J Mol Sci. 2021 Dec 8;22(24):13228. doi: 10.3390/ijms222413228. Int J Mol Sci. 2021. PMID: 34948026 Free PMC article. Review.
Cited by
-
A novel role of kallikrein-related peptidase 8 in the pathogenesis of diabetic cardiac fibrosis.Theranostics. 2021 Feb 20;11(9):4207-4231. doi: 10.7150/thno.48530. eCollection 2021. Theranostics. 2021. PMID: 33754057 Free PMC article.
-
The Role of NLRP3 Inflammasome Signaling on Arrhythmias in Diabetes.J Inflamm Res. 2022 Dec 29;15:6883-6889. doi: 10.2147/JIR.S390310. eCollection 2022. J Inflamm Res. 2022. PMID: 36600995 Free PMC article. Review.
-
P2X7 Receptor and Heart Function in a Mouse Model of Systemic Inflammation Due to High Fat Diet.J Inflamm Res. 2022 Apr 14;15:2425-2439. doi: 10.2147/JIR.S356038. eCollection 2022. J Inflamm Res. 2022. PMID: 35444452 Free PMC article.
-
Gypenosides improve diabetic cardiomyopathy by inhibiting ROS-mediated NLRP3 inflammasome activation.J Cell Mol Med. 2018 Sep;22(9):4437-4448. doi: 10.1111/jcmm.13743. Epub 2018 Jul 11. J Cell Mol Med. 2018. PMID: 29993180 Free PMC article.
-
Ginsenoside Rg1 Alleviates Lipopolysaccharide-Induced Fibrosis of Endometrial Epithelial Cells in Dairy Cows by Inhibiting Reactive Oxygen Species-Activated NLRP3.Animals (Basel). 2023 Dec 1;13(23):3723. doi: 10.3390/ani13233723. Animals (Basel). 2023. PMID: 38067074 Free PMC article.
References
-
- Gaspar L, Kruzliak P, Komornikova A, et al Orthostatic hypotension in diabetic patients‐10‐year follow‐up study. J Diabetes Complications. 2016; 30: 67–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

