Gas-Phase Reactions of Dimethyl Disulfide with Aliphatic Carbanions - A Mass Spectrometry and Computational Study
- PMID: 29313204
- PMCID: PMC5838211
- DOI: 10.1007/s13361-017-1858-x
Gas-Phase Reactions of Dimethyl Disulfide with Aliphatic Carbanions - A Mass Spectrometry and Computational Study
Abstract
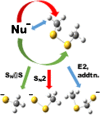
Ion-molecule reactions of Me2S2 with a wide range of aliphatic carbanions differing by structure and proton affinity values have been studied in the gas phase using mass spectrometry techniques and DFT calculations. The analysis of the spectra shows a variety of product ions formed via different reaction mechanisms, depending on the structure and proton affinity of the carbanion. Product ions of thiophilic reaction (m/z 47), SN2 (m/z 79), and E2 elimination - addition sequence of reactions (m/z 93) can be observed. Primary products of thiophilic reaction can undergo subsequent SN2 and proton transfer reactions. Gibbs free energy profiles calculated for experimentally observed reactions using PBE0/6-311+G(2d,p) method show good agreement with experimental results. Graphical Abstract ᅟ.
Keywords: Aliphatic carbanions; DFT calculations; Dimethyl disulfide; Gas-phase ion-molecule reactions; Thiophilic reaction.
Figures
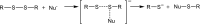
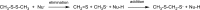

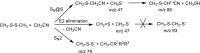
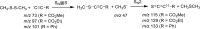
Similar articles
-
Gas-phase reactions of methyl thiocyanate with aliphatic carbanions - A mass spectrometry and computational study.Rapid Commun Mass Spectrom. 2016 Feb 15;30(3):393-9. doi: 10.1002/rcm.7449. Rapid Commun Mass Spectrom. 2016. PMID: 26754132
-
Scrubbing ions with molecules: kinetic studies of chemical noise reduction in mass spectrometry using ion-molecule reactions with dimethyl disulfide.Anal Chem. 2007 Jun 1;79(11):4006-12. doi: 10.1021/ac062369y. Epub 2007 May 8. Anal Chem. 2007. PMID: 17487975
-
Reactions of stabilized aliphatic carbanions with esters of formic acid in the gas phase.Eur J Mass Spectrom (Chichester). 2015;21(3):533-43. doi: 10.1255/ejms.1324. Eur J Mass Spectrom (Chichester). 2015. PMID: 26307733
-
Negative ion gas-phase chemistry of arenes.Mass Spectrom Rev. 2016 Jan-Feb;35(1):123-46. doi: 10.1002/mas.21467. Epub 2015 Apr 6. Mass Spectrom Rev. 2016. PMID: 25851641 Review.
-
Ion/ion chemistry of high-mass multiply charged ions.Mass Spectrom Rev. 1998 Nov-Dec;17(6):369-407. doi: 10.1002/(SICI)1098-2787(1998)17:6<369::AID-MAS1>3.0.CO;2-J. Mass Spectrom Rev. 1998. PMID: 10360331 Review.
Cited by
-
Conformational energies of reference organic molecules: benchmarking of common efficient computational methods against coupled cluster theory.J Comput Aided Mol Des. 2023 Dec;37(12):607-656. doi: 10.1007/s10822-023-00513-5. Epub 2023 Aug 19. J Comput Aided Mol Des. 2023. PMID: 37597063 Free PMC article. Review.
-
Plant-based seafood alternatives: Current insights on the nutrition, protein-flavour interactions, and the processing of these foods.Curr Res Food Sci. 2024 Sep 17;9:100860. doi: 10.1016/j.crfs.2024.100860. eCollection 2024. Curr Res Food Sci. 2024. PMID: 39381133 Free PMC article. Review.
References
-
- Grabowski JJ, Zhang L. Dimethyl disulfide: anion-molecule reactions in the gas phase at 300 K. J. Am. Chem. Soc. 1989;111:1193–1203. doi: 10.1021/ja00186a005. - DOI
-
- Lee JK, Grabowski JJ. Anion structure determination in the gas phase: chemical reactivity as a probe. J. Org. Chem. 1996;61:9422–9429. doi: 10.1021/jo961463j. - DOI
-
- Bachrach SM, Hayes JM, Dao T, Mynar JL. Density functional theory gas- and solution-phase study of nucleophilic substitution at di- and trisulfides. Theor. Chem. Acc. 2002;107:266–271. doi: 10.1007/s00214-002-0323-4. - DOI
-
- Bachrach SM, Mulhearn DC. Nucleophilic substitution at sulfur: S(N)2 or addition-elimination? J. Phys. Chem. 1996;100:3535–3540. doi: 10.1021/jp953335p. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

