Downregulation of miR-221-3p and upregulation of its target gene PARP1 are prognostic biomarkers for triple negative breast cancer patients and associated with poor prognosis
- PMID: 29312562
- PMCID: PMC5752475
- DOI: 10.18632/oncotarget.21561
Downregulation of miR-221-3p and upregulation of its target gene PARP1 are prognostic biomarkers for triple negative breast cancer patients and associated with poor prognosis
Abstract
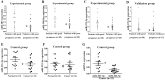
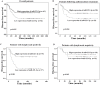
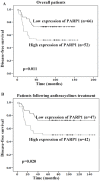
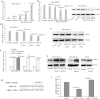
The purpose of this study was to identify microRNAs (miRNAs) closely associated with the prognosis of triple-negative breast cancer (TNBC) and their possible targets. This study recruited 125 early-stage TNBC patients, including 40 cases in the experimental group (20 cases with poor prognoses vs. 20 cases with good prognoses) and 85 cases in the validation group (27 cases with poor prognoses vs. 58 cases with good prognoses). In the experimental group, miRNA microarray showed 34 differentially expressed miRNAs in patients with different prognoses. We selected 5 miRNAs for validation. The differential expression of miR-221-3p was further verified in the experimental and validation groups using real-time polymerase chain reaction (PCR). High miR-221-3p expression was associated with better 5-year disease-free survival (DFS) (HR = 0.480; 95% CI, 0.263-0.879; p = 0.017) of TNBC patients. High expression of its target gene PARP1 predicted poorer 5-year DFS (HR = 2.236, 95% CI, 1.209-4.136, p = 0.010). MiR-221-3p down-regulated PARP1 by targeting its 3'-untranslated region. In conclusion, low miR-221-3p expression may contribute to the poor outcome of TNBC patients through regulating PARP1. MiR-221-3p likely plays a role as a PARP1 inhibitor by directly regulating PARP1 expression, thereby affecting the prognoses of TNBC patients.
Keywords: PARP1; miR-221-3p; microRNA; prognosis; triple negative breast cancer.
Conflict of interest statement
CONFLICTS OF INTEREST No potential conflicts of interest are disclosed.
Figures

Similar articles
-
miR-629-3p may serve as a novel biomarker and potential therapeutic target for lung metastases of triple-negative breast cancer.Breast Cancer Res. 2017 Jun 19;19(1):72. doi: 10.1186/s13058-017-0865-y. Breast Cancer Res. 2017. PMID: 28629464 Free PMC article.
-
MicroRNA-455-3p promotes invasion and migration in triple negative breast cancer by targeting tumor suppressor EI24.Oncotarget. 2017 Mar 21;8(12):19455-19466. doi: 10.18632/oncotarget.14307. Oncotarget. 2017. PMID: 28038450 Free PMC article.
-
miR-105/93-3p promotes chemoresistance and circulating miR-105/93-3p acts as a diagnostic biomarker for triple negative breast cancer.Breast Cancer Res. 2017 Dec 19;19(1):133. doi: 10.1186/s13058-017-0918-2. Breast Cancer Res. 2017. PMID: 29258605 Free PMC article.
-
Tumor tissue microRNA expression in association with triple-negative breast cancer outcomes.Breast Cancer Res Treat. 2015 Jul;152(1):183-191. doi: 10.1007/s10549-015-3460-x. Epub 2015 Jun 11. Breast Cancer Res Treat. 2015. PMID: 26062749 Free PMC article.
-
The Role of miRNAs in the Prognosis of Triple-Negative Breast Cancer: A Systematic Review and Meta-Analysis.Diagnostics (Basel). 2022 Dec 30;13(1):127. doi: 10.3390/diagnostics13010127. Diagnostics (Basel). 2022. PMID: 36611419 Free PMC article. Review.
Cited by
-
Identification of Cell-Free Circulating MicroRNAs for the Detection of Early Breast Cancer and Molecular Subtyping.J Oncol. 2019 Aug 8;2019:8393769. doi: 10.1155/2019/8393769. eCollection 2019. J Oncol. 2019. PMID: 31485228 Free PMC article.
-
GPER1 and microRNA: Two Players in Breast Cancer Progression.Int J Mol Sci. 2020 Dec 24;22(1):98. doi: 10.3390/ijms22010098. Int J Mol Sci. 2020. PMID: 33374170 Free PMC article. Review.
-
Recent advances of transcriptomics and proteomics in triple-negative breast cancer prognosis assessment.J Cell Mol Med. 2022 Mar;26(5):1351-1362. doi: 10.1111/jcmm.17124. Epub 2022 Feb 11. J Cell Mol Med. 2022. PMID: 35150062 Free PMC article. Review.
-
Hsa-miR-221-3p promotes proliferation and migration in HER2-positive breast cancer cells by targeting LASS2 and MBD2.Histol Histopathol. 2022 Nov;37(11):1099-1112. doi: 10.14670/HH-18-483. Epub 2022 Jun 23. Histol Histopathol. 2022. PMID: 35734966
-
NLRP3 inflammasome inactivation driven by miR‑223‑3p reduces tumor growth and increases anticancer immunity in breast cancer.Mol Med Rep. 2019 Mar;19(3):2180-2188. doi: 10.3892/mmr.2019.9889. Epub 2019 Jan 23. Mol Med Rep. 2019. PMID: 30747211 Free PMC article.
References
-
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. - PubMed
-
- Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. The Lancet Oncology. 2007;8:235–244. - PubMed
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

